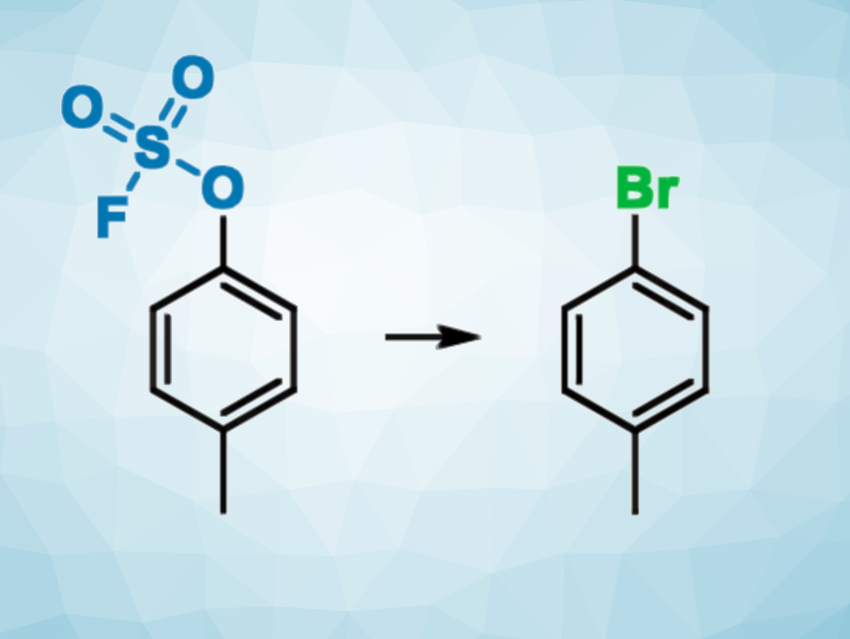
Aryl and alkenyl halides are widely used as key intermediates in organic synthesis. One method that can be used to prepare these compounds is the conversion of the corresponding aryl or vinyl alcohols, which are more readily available, into their halogenated analogues. However, this approach usually requires harsh reaction conditions and is often limited to the synthesis of only one or two types of halogenated compounds (with Cl, Br, or I).
Frédéric R. Leroux, University of Strasbourg, France, and colleagues have developed an approach for the efficient conversion of phenols to aryl halides via an activation with gaseous sulfuryl fluoride (SO2F2), leading to the corresponding fluorosulfonates. The activation is followed by a ruthenium-catalyzed conversion using simple halide sources (NaCl, LiBr, or LiI) to obtain the desired products. The team used Cp*RuCl(cod) or [RuCp*(MeCN)3]PF6 as catalysts (Cp* = 1,2,3,4,5-pentamethylcyclopentadiene, cod = cyclooctadiene). The method can be extended to the synthesis of vinyl halides from the corresponding carbonyl compounds via a fluorosulfonate derivative of the corresponding enol (pictured below).
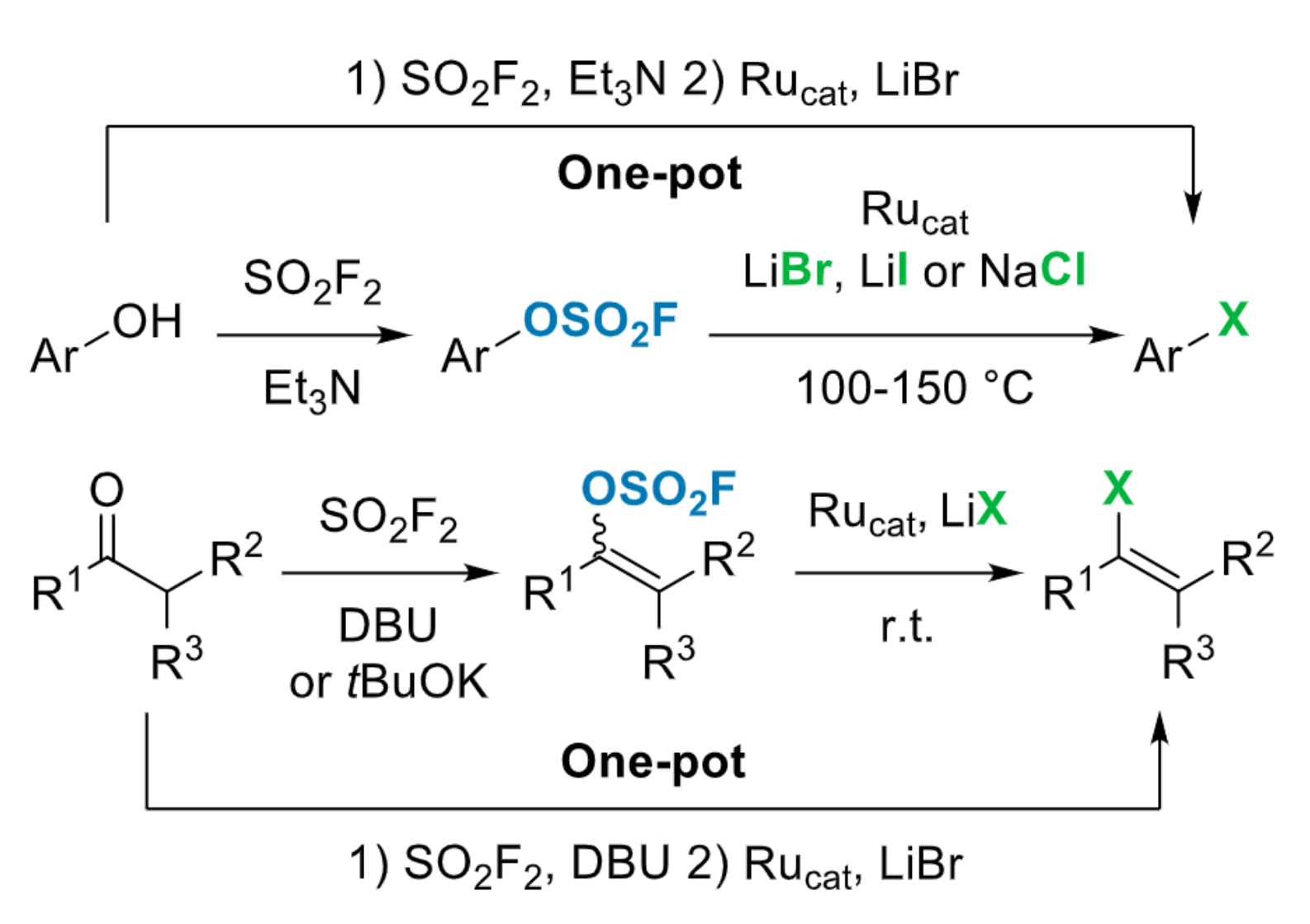
The approach allows a one-pot transformation, generates little waste, and gives access to chlorinated, brominated, or iodinated derivatives. Overall, the work provides versatile and efficient access to various aryl or vinyl halides.
- Ruthenium‐catalyzed Synthesis of Aryl and Alkenyl Halides from Fluorosulfonates,
Clotilde Plaçais, Sherif J. Kaldas, Morgan Donnard, Armen Panossian, David Bernier, Sergii Pazenok, Frederic R. Leroux,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301420