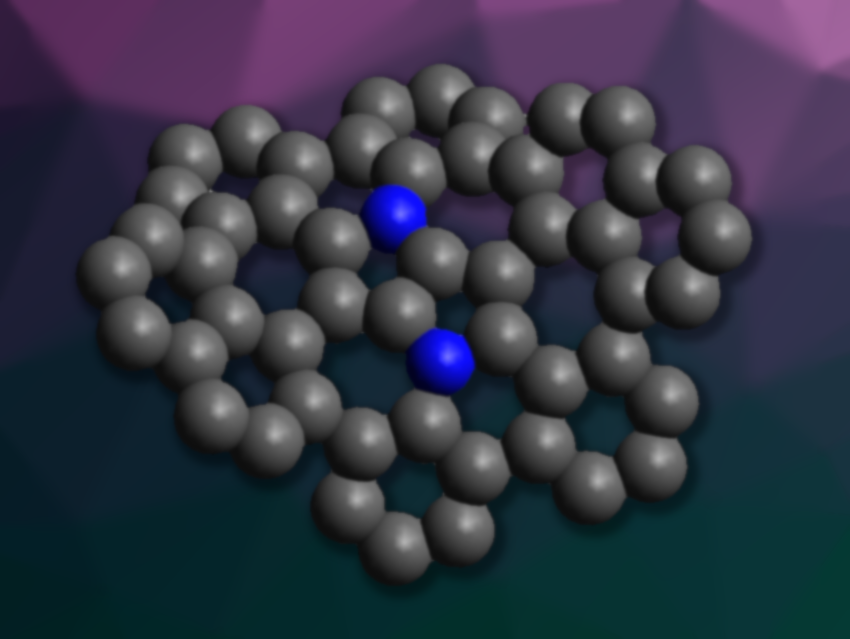
Carbon can be used to build curved molecules with interesting properties, such as fullerenes or nanotubes. Planar graphene is made from six-membered rings. Introducing five-membered rings and larger, e.g., seven-membered, rings into graphene subunits can introduce curvatures and result in saddle-like shapes. The addition of heteroatoms such as nitrogen can be used to tune the properties of the resulting structures. However, examples of saddle-shaped aza-nanographenes are rare.
Michał K. Cyrański, University of Warsaw, Poland, Daniel T. Gryko, Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, and colleagues have synthesized a saddle-shaped aza-nanographene containing a 1,4-dihydropyrrolo[3,2-b]pyrrole (DHPP) core (central structure pictured). The structure features two central pentagons with four adjacent heptagons.
The team first prepared a DHPP precursor, starting from 2-bromo-4-(tert-butyl)-6-chlorobenzaldehyde, 2-(naphthalen-2-yl)-4-octylaniline, and butane-2,3-dione. This reaction gave a bromo- and chloro-substituted tetra-aryl pyrrolo[3,2-b]pyrrole derivative, with two 2-(naphthalen-2-yl)phenyl groups and two phenyl units. An intramolecular direct arylation, a double Scholl reaction, and a photo-induced radical cyclization were then used to close the remaining rings and give the desired saddle-shaped nanographene.
Overall, the product was prepared in four steps with a yield of 8 % and then characterized using NMR spectroscopy and high-resolution mass spectrometry (MS). Cyclic voltammetry measurements showed that it can undergo three fully reversible oxidation steps. According to the researchers, it is the first nanographene with a unique 7–7–5–5–7–7 topology.
- Saddle-shaped aza-nanographene with multiple odd-membered rings,
Maciej Krzeszewski, Lukasz Dobrzycki, Andrzej L. Sobolewski, Michal Ksawery Cyranski, Daniel T. Gryko,
Chem. Sci. 2023.
https://doi.org/10.1039/D2SC05858H