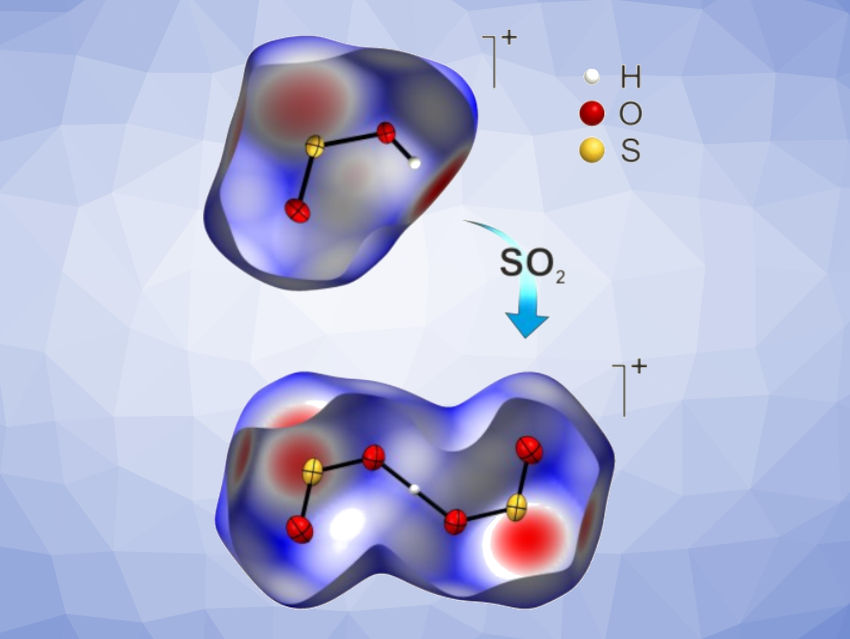
Sulfur dioxide is an important solvent in superacid chemistry, e.g., as the medium for reactions with so-called “magic acid” (FSO3H/SbF5) or as a solvent for carborane acids. In this context, protonated species based on SO2 have been detected and studied using quantum-chemical calculations. However, the properties and structure of protonated SO2 had not been studied in-depth so far.
Andreas J. Kornath, Ludwig-Maximilian University of Munich, Germany, and colleagues have synthesized and isolated hemi- and monoprotonated SO2-based cations (pictured) in the form of their Sb2F11− salts. The team used [FS(OX)2][SbF6] (X = H,D) as a precursor, which can be obtained from SO2 and SbF5 in anhydrous hydrogen fluoride.
This compound was recrystallized in aprotic solvents at low temperatures. In SO2, the researchers observed the formation of hemiprotonated SO2 as [(SO2)2H][Sb2F11] and the corresponding deuterated species. In 1,1,1,2-tetrafluoroethane, monoprotonated sulfur dioxide was formed from the deuterated precursor as the salt [OSOD][Sb2F11].
The products were characterized using Raman spectroscopy and single-crystal X-ray structure analysis. For hemiprotonated SO2, the work represents the first observation of the respective ν(SO) and δ(OSO) vibrational frequencies.
- Synthesis and Structure of Protonated Sulfur Dioxide,
Christoph Jessen, Andreas J. Kornath,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202401953