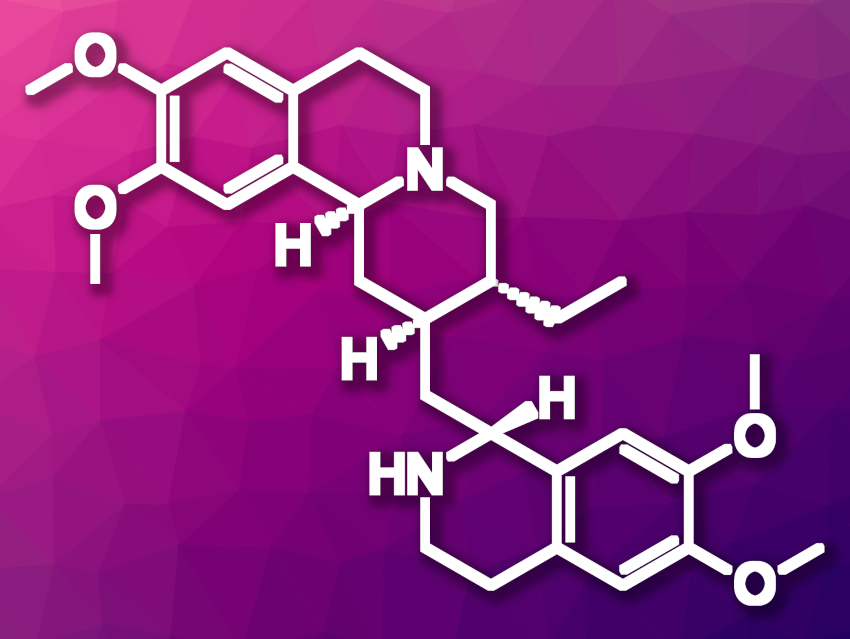
(−)-Emetine (pictured) is a natural product found as an active ingredient in ipecac root. Syrup of ipecac has been used in the past as an emetic, i.e., a substance that induces vomiting, and as an expectorant, i.e., a type of cough medicine. Emetine has also been studied for possible anticancer properties. However, emetine’s toxicity and side effects make its use challenging. Using emetine as a starting point for further drug development could be promising.
Masatoshi Yamada, SPERA PHARMA, Inc., Osaka, Japan, and colleagues have developed a scalable asymmetric total synthesis of (−)-emetine, with the aim of producing suitable quantities for ongoing drug development. The team first prepared 6,7-dimethoxy-3,4-dihydroisoquinoline starting from 1.41 kg of homoveratrylamine. The product then underwent an asymmetric allylation. The allyl-containing intermediate was converted to an (E)-alkene derivative using olefin metathesis, followed by a Michael addition with methyl vinyl ketone and a cyclization. The obtained benzoquinolizidine ketone was reduced, followed by tosyl protection and deoxygenation.
The ester group of the resulting intermediate was saponified, followed by an amidation with a second homoveratrylamine unit. The amide was then converted into a cyclic imine. Finally, an asymmetric transfer hydrogenation and a hydrochloride formation gave 237 g of emetine dihydrochloride.
The synthetic route features a total of 13 steps (10 isolated steps) and was performed without chromatographic purification. The desired (−)-emetine·2HCl was obtained in 12 % overall yield. According to the researchers, the synthesis should be suitable for the production of larger quantities of emetine for drug development.
- Efficient and Scalable Asymmetric Total Synthesis of (−)-Emetine with Pharmaceutical Grade Quality; First Multigram Scale Synthesis,
Masatoshi Yamada, Kazuki Azuma, Iori Takizawa, Yuki Ejima, Mitsuhisa Yamano, Kimio Satoh, Takayuki Doi, Hirofumi Ueda, Hidetoshi Tokuyama,
Org. Process Res. Dev. 2023.
https://doi.org/10.1021/acs.oprd.2c00355