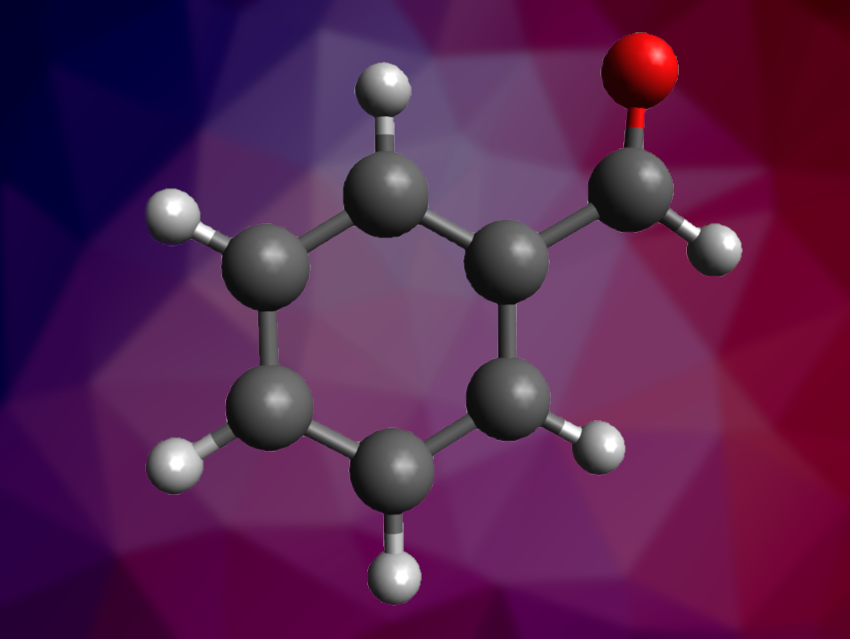
The oxidation of toluene to benzaldehyde is a useful reaction to obtain value-added oxygenated products. However, toluene can be difficult to oxidize selectively. Thus, benzaldehyde production from toluene usually involves multiple steps and environmentally harmful reagents or suffers from low selectivity. Photocatalytic oxidation using O2 could be a greener alternative but requires suitable catalysts.
Zelong Li, Lanzhou University, China, Can Li, Lanzhou University and Dalian National Laboratory for Clean Energy, China, and colleagues have developed a photocatalyst for the selective oxidation of toluene to benzaldehyde. The catalyst consists of a small amount (1 mol%) of amorphous BiOCl nanosheets loaded onto TiO2.
The developed catalyst showed high performance for the oxidation of toluene to benzaldehyde, with a toluene conversion of 10 % and a benzaldehyde selectivity of up to 85 %. In comparison, “naked” TiO2 provides only 3 % toluene conversion and leads to overoxidation, with a benzaldehyde selectivity of only 51 %. The team states that abundant surface oxygen vacancies in amorphous BiOCl facilitate the adsorption and activation of O2 and that BiOCl shows faster benzaldehyde desorption than TiO2, thus improving activity and selectivity.
- Achieving High Selectivity in Photocatalytic Oxidation of Toluene on Amorphous BiOCl Nanosheets Coupled with TiO2,
Hao Wang, Chen Cao, Dongfeng Li, Yongxin Ge, Ruotian Chen, Rui Song, Wensheng Gao, Xiuli Wang, Xintan Deng, Hongjun Zhang, Bangjiao Ye, Zelong Li, Can Li,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c05237