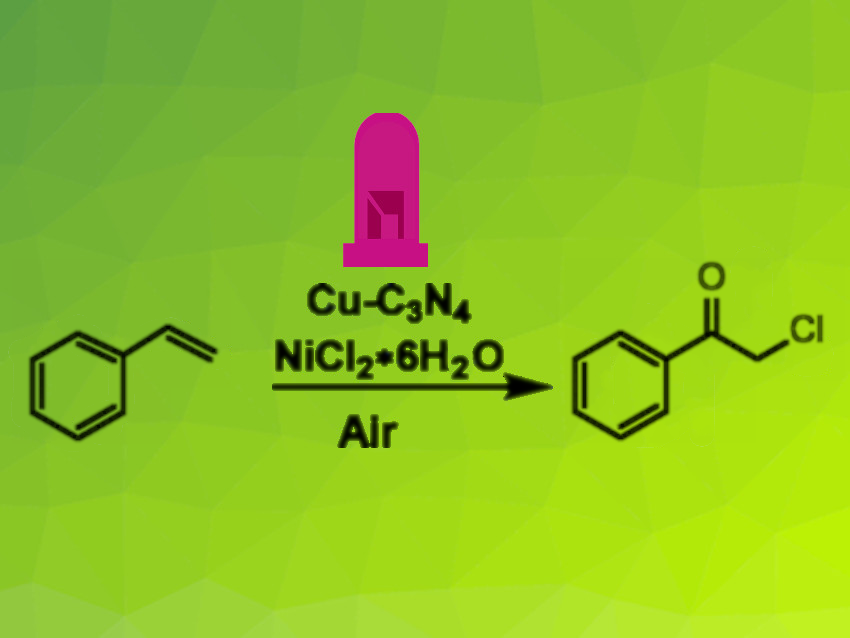
Ketones with halogen atoms in the α-position are useful synthetic building blocks for the preparation of complex organic molecules. They are usually synthesized by the direct halogenation of ketones. The use of elemental halogens or halogen radical precursors like N-halosuccinimides in these reactions is problematic at an industrial scale. Elemental halogens are toxic and corrosive, while the use of succinimides generates large amounts of waste. These problems can be reduced by processes that use inorganic chloride salts directly. One approach towards this is the photocatalytic oxidation of alkenes in the presence of chloride salts.
Emma Richards, Cardiff University, UK, Ren Su, Soochow University, Suzhou, China, and colleagues have developed a photochemical synthesis of α-haloketones from styrene derivatives using nickel chloride as the halogen source, a copper-modified graphitic carbon nitride catalyst (Cu-C3N4) as the photocatalyst, and oxygen from air as the oxidant. The reaction was optimized for the conversion of styrene to 2-chloroacetophenone (pictured). Nickel chloride hexahydrate was the most efficient halogen source, and a mixture of ethyl acetate and isopropanol as the solvent gave the highest selectivity. The presence of isopropanol as a hydrogen source for the formation of water was essential. A 410 nm LED was used as the light source. When sunlight was used instead, full conversion was of styrene was achieved, but the selectivity was reduced.
Styrene derivatives with electron-withdrawing groups were selectively converted to the target products in high yields. Electron-withdrawing substituents reduced the selectivity. The synthesis of α-bromoketones with nickel bromide was also successful. When irradiated with 410 nm LED light, the carbon nitride catalyst activates dioxygen and generates oxygen-centered radicals. The copper-modified catalyst allows the selective generation of •OOH radicals, which are suitable for the desired reaction. The formation of chlorine radicals from nickel chloride hexahydrate is relatively slow, which also enhances the selectivity. Therefore, the combination of Cu-C3N4 and nickel chloride hexahydrate gave the best results under the tested reaction conditions.
- Copper Cocatalyst Modulated Radical Generation for Selective Heterogeneous Photosynthesis of α-Haloketones,
Feiyu Han, Dongsheng Zhang, Sofia Salli, Jiani Ye, Yongwang Li, Federico Rosei, Xiao-Dong Wen, Hans Niemantsverdriet, Emma Richards, Ren Su,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c05189