Rare-earth metals are indispensable for many technical products, from smartphones, laptops, batteries, electromotors, and wind turbines, to catalysts. A Japanese team has introduced a molecular “cage” with “caps” that can be used to selectively “confine” certain rare-earth-metal ions for isolation or recycling.
Rare-Earth Elements
The rare-earth elements include 17 metals: scandium, yttrium, lanthanum, and the lanthanides, the 14 elements that follow after lanthanum in the periodic table, including neodymium and europium. The name is misleading because the rare-earth metals are not actually rare. They are everywhere in the environment but are highly dispersed and bound in minerals (“earths”); large deposits are rare.
Reclaiming these elements from electronic waste is becoming more important. Some microorganisms have been discovered that contain enzymes with rare-earth metals. These could be useful in extraction and reclamation and provide inspiration for the use of rare-earth metals as catalysts.
Rare-earth-metal ions are also found in bodies of water and in effluent. However, they are hard to separate individually from aqueous solutions. One reason for this is that they are usually hydrated, meaning that they are bound to water molecules. Their states of hydration are different and may change. This makes identification and isolation of the ions through binding to ligands more difficult.
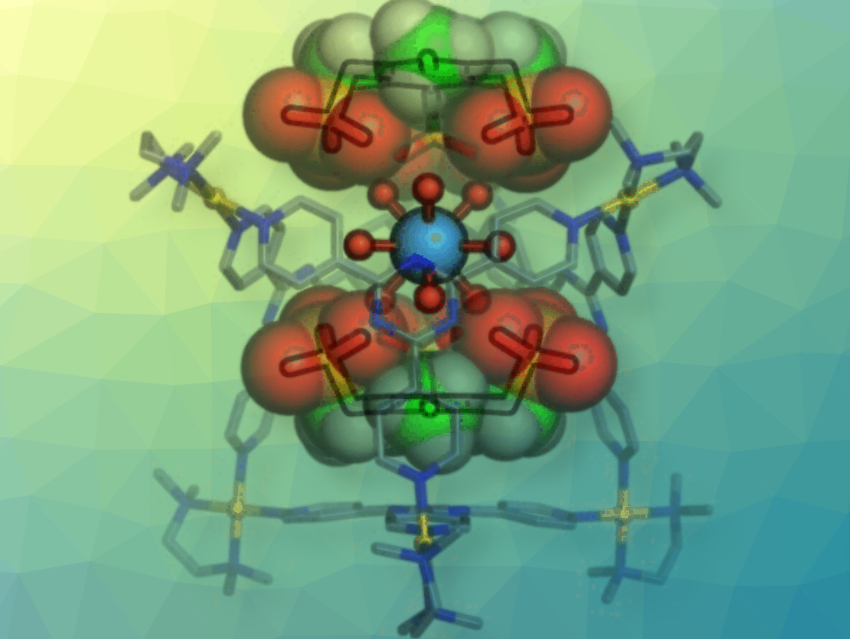
Encapsulation of a Hydrated Rare-Earth Metal Ion
A team led by Makoto Fujita at the University of Tokyo, Japan, and the Institute for Molecular Science, Aichi, Japan has managed to “confine” the hydrated forms of trivalent ions of a series of rare-earth metals in closed “cages”. Each cage molecule consists of four organic ligands shaped like triangular “plates” that are connected by their tips to six palladium ions to make an octahedral cage with two large openings. The rare-earth-metal ion fits into the cage with its nine bound water molecules.
The critical feature of the cage are its two “caps” that cover the openings. These are planar molecules with three negatively charged binding arms that bind to the rare-earth-metal ion’s water molecules through hydrogen bridges. In addition, they are held tight by electrostatic interactions with the positively charged palladium ions in the cage.
Not all rare-earth-metal ions are captured equally well by this system. Subtle differences in their radii and preferred modes of hydration determine how well they fit into the cages: lanthanum and the early lanthanides, such as europium, are bound significantly more strongly than the later lanthanides, like ytterbium. Scandium, for example, only has six water molecules bound to it and cannot find a stable position within the cage. It is thus barely held in place.
Confinement of hydrophilic metal species in a closed cavity could be an approach for the isolation of rare-earth metals, as well as for the development of novel catalysts analogous to metal-containing enzymes (metallozymes) in microorganisms.
- Selective Confinement of Rare-Earth-Metal Hydrates by a Capped Metallo-Cage under Aqueous Conditions,
Ryosuke Tabuchi, Hiroki Takezawa, Makoto Fujita,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202208866


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
