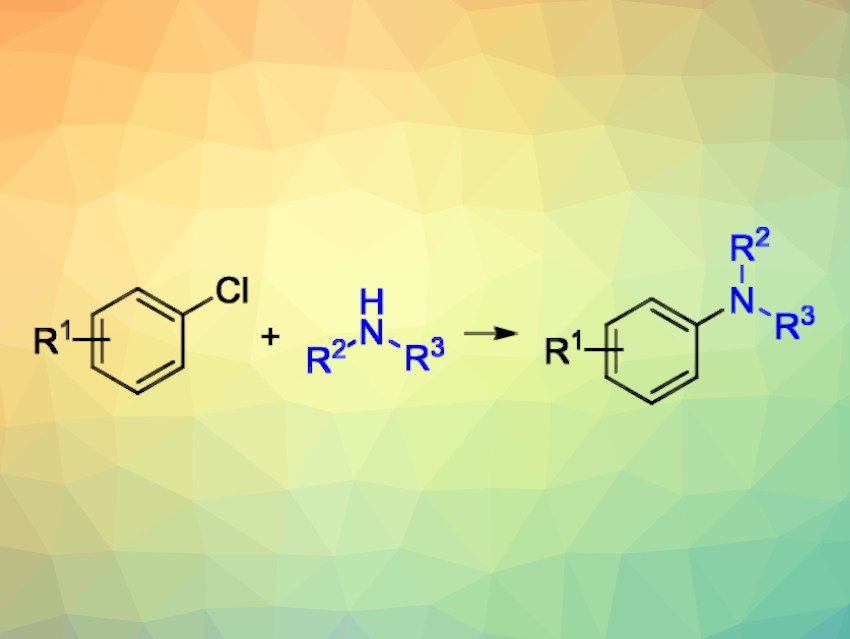
C–N cross-coupling reactions between (hetero)aryl halides and amines under palladium catalysis, i.e., Buchwald–Hartwig aminations, are widely used in organic synthesis. Nickel as a more abundant metal could replace the expensive palladium in some reactions. Nickel complexes with N-heterocyclic carbenes (NHCs) could be useful in this context. However, the use of well-defined Ni(0)/NHC complexes or complexes generated in situ from Ni(0) precursors such as the widely used Ni(COD)2 (COD = 1,5-cyclooctadiene) can be inconvenient to due to the cost or air-sensitivity of these compounds.
Victor M. Chernyshev, Platov South-Russian State Polytechnic University (NPI), Novocherkassk, Russia, Valentine P. Ananikov, Platov South-Russian State Polytechnic University and Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, and colleagues have developed a simple and efficient protocol for Ni-catalyzed C–N cross-coupling reactions of aryl chlorides with (hetero)aryl-and alkyl amines. The team used a catalytic system based on nickel NHC complexes generated in situ from cheap, bench-stable precursors: a NiCl2 adduct with pyridine, the imidazolium precursor of the NHC, and sodium tert-butoxide. All catalyst precursors and reagents can be loaded in air. The researchers propose that the Ni(0)/NHC active species formation involves a Ni(II) to Ni(0) reduction under participation of the NHC ligands and tert-butoxide anions.
Using this catalytic system, the team performed reactions of various aryl- and alkylamines, as well as some amides, with a broad range of aryl chlorides. They obtained the desired cross-coupling products in yields of 58–95 %. Overall, the protocol could serve as a simple alternative to methods relying on the use of air-sensitive Ni(COD)2 or expensive well-defined Ni/NHC precatalysts.
- A Simple Protocol for the C‐N Cross‐Coupling of Aryl Chlorides with Amines Applying Ni/NHC Catalysis,
Oleg Khazipov, Anastasia Pyatachenko, Victor Chernyshev, Valentine P. Ananikov,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300466