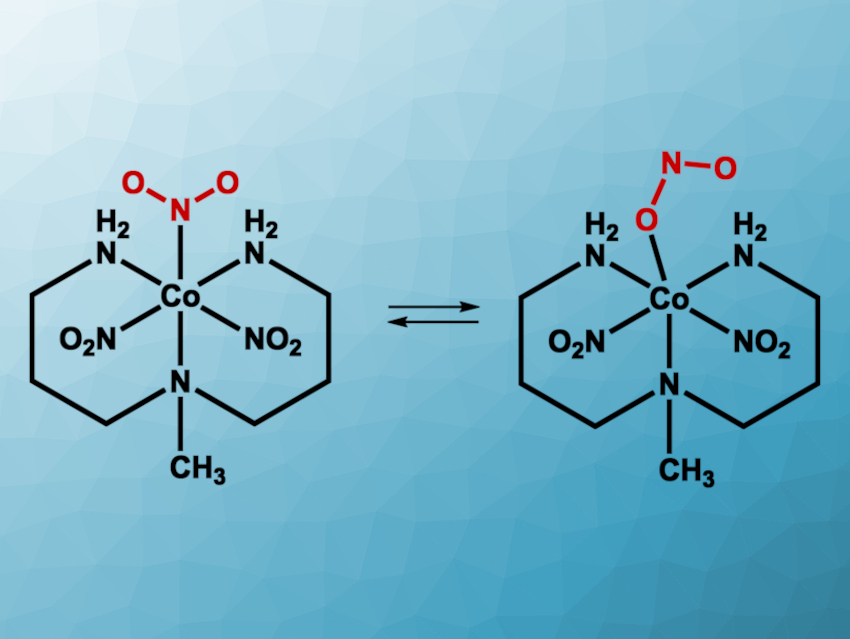
Photoswitchable materials can have applications, for example, in optoelectronics or sensing. They can change their structure under irradiation with light, e.g., via the photoisomerization of a C=C bond. Inorganic compounds can also serve as photoswitches. For example, transition-metal complexes with ambidentate ligands—ligands that can bind via different atoms—could potentially act as photoswitches. Nitrite ligands are ambidentate: They can bind in a nitro (–NO2) or a nitrito (–ONO) configuration.
Katarzyna N. Jarzembska, University of Warsaw, Poland, and colleagues have found that the simple cobalt complex [Co(Me-dpt)(NO2)3] (Me-dpt = 3,3′-diamino-N-methylpropanediamine) can act as a photoswitch at room temperature with a maximum nitro-to-nitrito conversion of about 55 % (pictured). According to the researchers, this is the most efficient room-temperature photoswitch based on a transition-metal nitro complex reported so far.
The complex was prepared from CoCl2 hexahydrate, 3,3′-diamino-N-methylpropanediamine, and KNO2. X-ray diffraction showed that the majority of the product exists as the nitro isomer in the solid state (about 88 %). The team then irradiated the samples with different wavelengths of light and collected solid-state UV-Vis and infrared (IR spectra) at room temperature. They found that under irradiation at ca. 470 nm, there is a notable increase of nitrito-type ligands. Light with a wavelength of ca. 570–660 nm triggers a back-conversion to the nitro-type isomer. Thus, the work is interesting for the development of reversible, room-temperature photoswitches.
- An optically reversible room-temperature solid-state cobalt(iii) photoswitch based on nitro-to-nitrito linkage isomerism,
Krystyna A. Deresz, Radosław Kamiński, Sylwia E. Kutniewska, Adam Krówczyński, Dominik Schaniel, Katarzyna N. Jarzembska,
Chem. Commun. 2022.
https://doi.org/10.1039/D2CC05134F