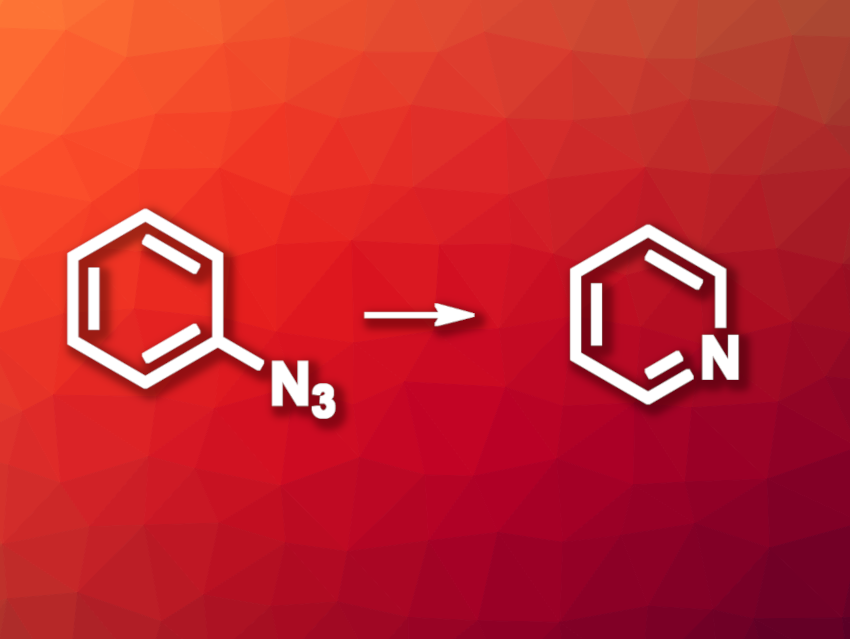
Replacing just one atom in an organic compound with another can be useful, e.g., in drug development. For example, replacing an aromatic carbon atom with a nitrogen atom to give a pyridine derivative can lead to drug candidates. Figuring out where this nitrogen’s optimal position might be is called a “nitrogen scan” in drug development. While this is simple on paper, it can be a challenge for synthetic chemistry.
Mark D. Levin, University of Chicago, IL, USA, and colleagues have developed a method for a site-selective carbon-to-nitrogen replacement in aromatic compounds. The team started from aryl azides, which were first photochemically converted to 2-amino-3H-azepine derivatives, i.e., seven-membered rings that contain a nitrogen atom. For this, they used ethylaminoethanol, and the reaction was performed under LED light in MeCN. The azepine-derived intermediate was then converted to the desired pyridine derivative using diazabicycloundecene (DBU) as a base and N-bromocaprolactam (NBC) as an oxidant in dioxane at 80 °C. N-ethyl oxazolidinone was formed as a byproduct, presumably from the ethylaminoethanol unit and the removed carbon atom after an oxidation step.
According to the team, the developed reaction can be used for nitrogen scans because the starting azides can be prepared from a variety of functionalized arenes and the reaction selectively replaces the carbon atoms at the ipso position. This enables predictable replacement reactions that are useful in medicinal chemistry, but could also have other applications.
- Aromatic nitrogen scanning by ipso-selective nitrene internalization,
Tyler J. Pearson, Ryoma Shimazumi, Julia L. Driscoll, Balu D. Dherange, Dong-Il Park, Mark D. Levin,
Science 2023.
https://doi.org/10.1126/science.adj5331
Update (October 16, 2023): The article originally contained a misspelled name. This has been corrected.