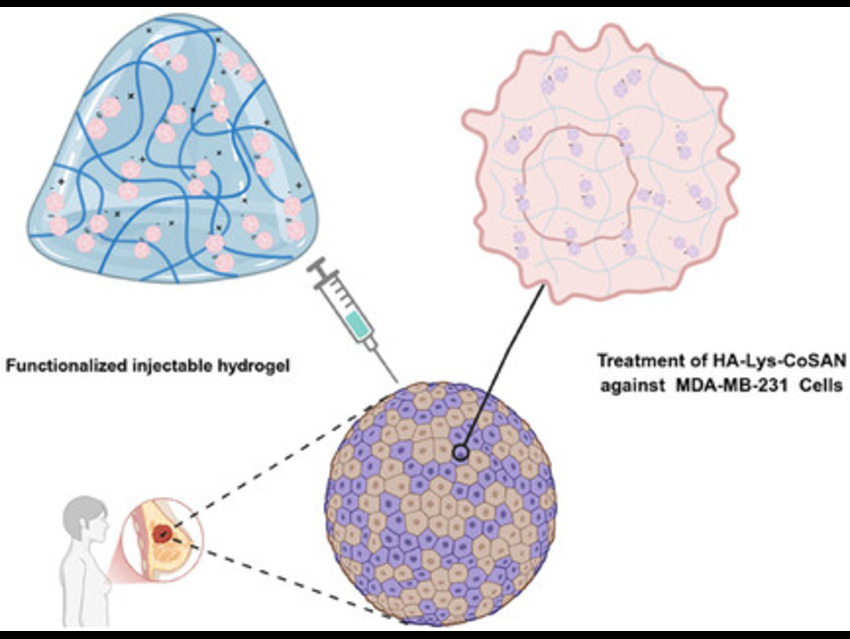
Triple-negative breast cancer (TNBC) is a highly aggressive form of cancer with limited treatment options. This study aims to develop a safe and effective drug delivery system that targets TNBC cells while minimizing harm to healthy cells. Pau Farràs, University of Galway, Ireland, and colleagues have investigated cobalt-based metallacarboranes as potential cancer therapies. The study introduces a new injectable hydrogel made from hyaluronic acid (HA) functionalized with lysine, capable of carrying cobalt metallacarborane (CoSAN) without altering its anticancer properties. This noncovalent loading preserves drug activity and allows controlled release, making it a promising platform for targeted cancer therapy.
The research involved creating a targeted drug delivery system by modifying hyaluronic acid (HA) with lysine using 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) in a buffered solution at pH 6 and 30 °C overnight. After removing the Boc protecting group with hydrochloric acid and trifluoroacetic acid, the exposed amine allowed cobalt metallacarborane (CoSAN) to bind electrostatically under acidic conditions.
The resulting HA-Lys-CoSAN composite was purified and lyophilized. Characterization via diffusion-ordered nuclear magnetic resonance (NMR) spectroscopy (DOSY), Fourier transform infrared spectroscopy (FTIR), energy-dispersive X-ray spectroscopy (EDX), and scanning electron microscope (SEM) imaging confirmed successful synthesis and uniform CoSAN distribution within the porous hydrogel. Biological testing showed strong anticancer activity against triple-negative breast cancer cells (MDA-MB-231) with low toxicity to healthy fibroblasts (HDF), and IC₅₀ values of 17 μM for HA-Lys-CoSAN and 23 μM for CoSAN. Stimulated Raman scattering microscopy confirmed efficient cellular uptake. Drug release studies using UV–Vis and dialysis revealed faster release at neutral pH and slower release under acidic conditions, making the system ideal for gradual drug delivery in tumor environments. Additionally, it achieves good cellular uptake comparable to free CoSAN, with signs of enhanced delivery efficiency due to the HA-based carrier system.
In summary, this study presents a smart, biocompatible drug delivery system with potential to improve TNBC treatment and possibly other cancers. Its ability to release drugs gradually in acidic environments while maintaining therapeutic potency opens doors for future clinical applications.
- Functionalizing Injectable Hydrogels with Cobalt-Based Metallacarboranes for Targeted Delivery in Triple-Negative Breast Cancer
Neville Murphy, Roberto González-Gómez, Nivethitha Ashok, Enda O’Connell, Howard Fearnhead, William J. Tipping, Karen Faulds, Wenming Tong, Abhay Pandit, Róisín M. Dwyer, Duncan Graham, Pau Farràs
ChemBioChem 2025
https://doi.org/10.1002/cbic.202500589