Covalent organic frameworks (COFs) are porous crystalline networks made from organic building blocks. Similarly to metal–organic frameworks (MOFs), they can be useful, e.g., in gas adsorption/separation, sensing, or catalysis. There is a subtype of flexible COFs that can undergo a reversible transformation between a porous and a non-porous state (similar to how a sponge can be compressed/decompressed to close/open its pores), so-called soft porous crystals (SPCs). SPCs can be useful in applications such as pressure-dependent gas adsorption.
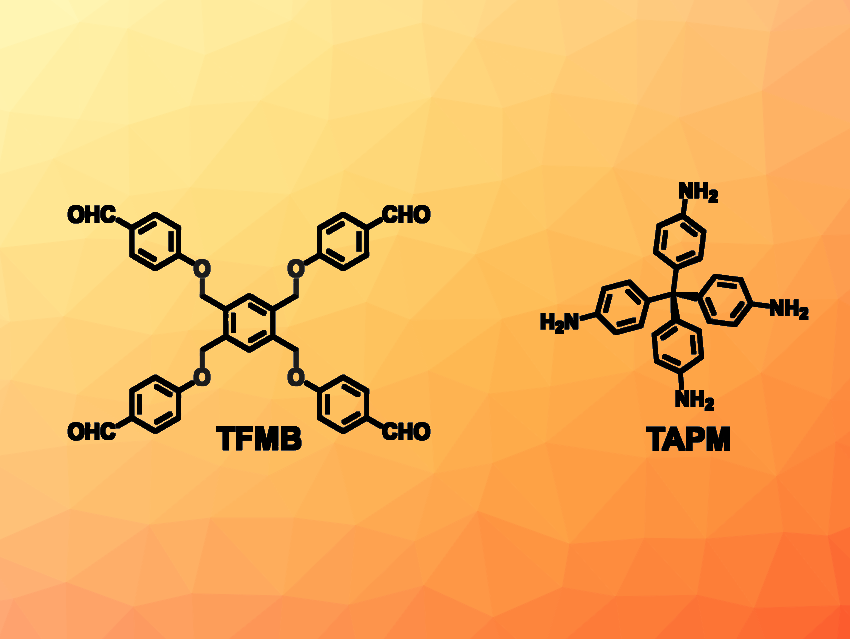
Cheng Wang, Wuhan University, China, and colleagues have developed a COF (called FCOF-5) that shows a reversible expansion and contraction upon the adsorption of certain gases, i.e., it exhibits a gas-triggered gate-opening behavior. The team prepared FCOF-5 from tetra(p-aminophenyl)methane (TAPM) and 1,2,4,5-tetrakis[(4-formylphenoxy)methyl]benzene (TFMB, both pictured) via a condensation reaction. The C–O single bonds in the TFMB building block allow for changes in conformation and provide the framework with a certain flexibility.
In the absence of gases, FCOF-5 is structurally contracted and has very small pore sizes. The team found that these ultramicopores do not allow for the diffusion of even N2. For CO2, the team observed almost no adsorption at low pressure but a sudden increase in adsorption at higher pressures. This sudden increase provides evidence for a phase transformation of the COF from a contracted to an expanded state—i.e., the pores “open up” upon the adsorption of CO2. According to the researchers, such a CO2-triggered gate-opening behavior had rarely been observed in 3D COFs so far.
The team also investigated the adsorption of C2H2, C2H4, and C2H6, as well as of C3 hydrocarbons (propyne, propylene, and propane) and found a similar guest-triggered gate-opening behavior. Due to differences in the gate-opening pressures, FCOF-5 can, for example, be used to separate trace amounts of C3H4 from C3H4/C3H6 mixtures.
- Gas-Triggered Gate-Opening in a Flexible Three-Dimensional Covalent Organic Framework,
Xiaoling Liu, Zhifang Wang, Ya Zhang, Na Yang, Bo Gui, Junliang Sun, Cheng Wang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c01331