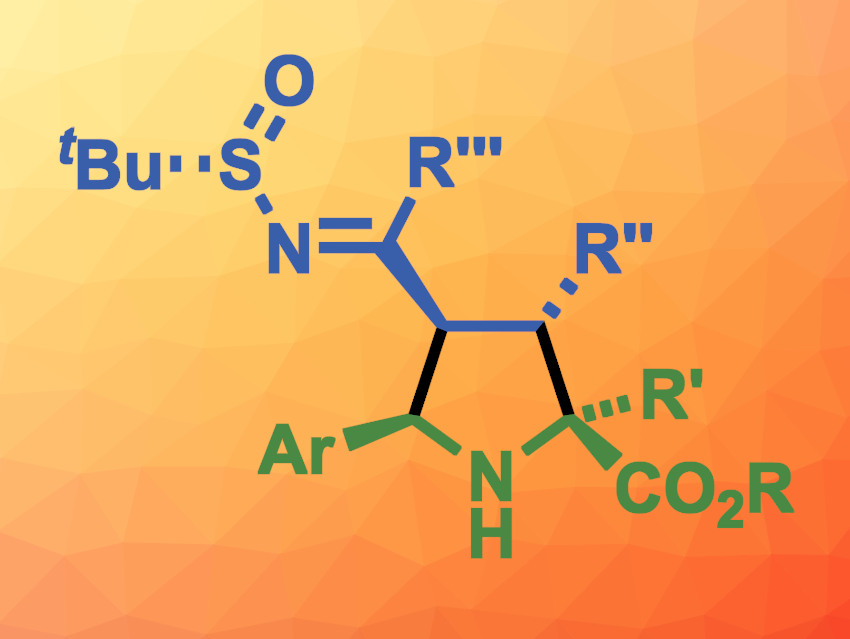
Stereoselective 1,3-dipolar cycloadditions can be useful in organic synthesis and can be used to introduce four stereogenic centers at once. Cycloadditions of azomethine ylides with alkenes, for example, can be used to prepare pyrrolidines. Chiral tert-butanesulfinyl imines could be interesting as reactants in this context, but they usually react at the N=C bond and generally not at the conjugated C=C bond.
María de Gracia Retamosa, José M. Sansano, University of Alicante, Spain, and colleagues have developed a method for the stereoselective synthesis of densely substituted pyrrolidines (general structure pictured) via a [3 + 2] cycloaddition reaction between chiral N–tert-butanesulfinylazadienes and azomethine ylides, which were generated in situ from imino esters. The team used a wide variety of imino esters and N–tert-butanesulfinyl imines as substrates, Ag2CO3 as a catalyst, and toluene as the solvent. The reactions were performed at room temperature.
Using this approach, the team obtained a family of densely substituted pyrrolidines with up to four stereogenic centers in the pyrrolidine ring in moderate to good yields and good to excellent regio- and diastereoselectivities. The products can be further transformed, e.g., into valuable proline derivatives that can be used as organocatalysts.
- Stereoselective Synthesis of Densely Substituted Pyrrolidines via a [3 + 2] Cycloaddition Reaction between Chiral N-tert-Butanesulfinylazadienes and Azomethine Ylides,
Ester Blanco-López, Francisco Foubelo, María de Gracia Retamosa, José M. Sansano,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02572