Heterocyclic compounds with phosphor atoms, i.e., phosphaheterocycles, have applications, e.g., in pharmaceutical chemistry, agrochemistry, and catalysis. Phosphinolactones, for example, are phosphorus analogs of lactones and can be useful, e.g., in drug development. δ-Phosphinolactones, in particular, are potential biologically active compounds. There is a limited range of options for the synthesis of δ-phosphinolactones.
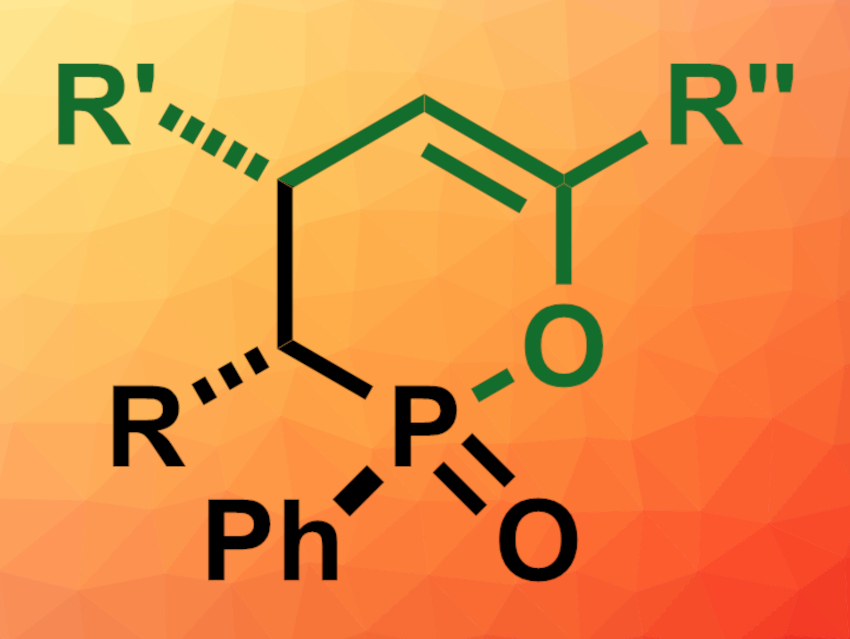
Jiaxi Xu, Beijing University of Chemical Technology, China, and colleagues have developed an efficient, stereospecific synthesis of δ-phosphinolactones (general product structure pictured) via the direct [2 + 4] annulation of alkyl(phenyl)phosphinic chlorides and α,β-enones in the presence of lithium hexamethyldisilazide (LiHMDS). The team reacted alkyl(phenyl)phosphinic chlorides with different substituents with a range of enones in the presence of LIHMDS at room temperature, using tetrahydrofuran (THF) as the solvent.
The desired δ-phosphinolactone derivatives were obtained in mostly good to excellent yields with diastereospecificity. The reaction can be performed on a gram scale. The team proposes a reaction mechanism that involves a deprotonation of the alkyl(phenyl)phosphinic chloride, followed by an intramolecular Michael addition with the enone and a nucleophilic attack at the phosphorus atom. The products can be further transformed.
- [2 + 4] Annulation of Alkyl(phenyl)phosphinic Chlorides with Enones: Stereoselective Synthesis of δ-Phosphinolactones,
Xin Yuan, Xuan Ke, Jiaxi Xu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03878


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
