Phosphine-based ligands are widely used, e.g., in catalysis. They are generally straightforward to synthesize, and their properties can be tuned by modifying the substituents. Triarylphosphines are often employed, but trialkylphosphines can usually provide stronger electron-donating properties. Triarylphosphines with stronger donor abilities could be a useful addition to the “ligand toolbox”.
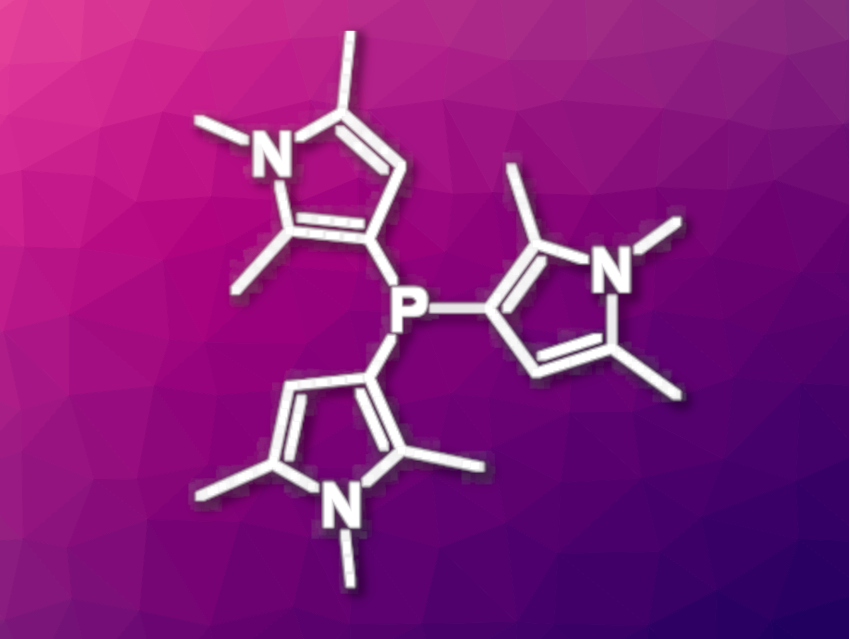
Fabian Dielmann, Universitäy of Innsbruck, Austria, and colleagues have synthesized 1,2,5-trimethylpyrrolyl phosphines (example pictured) as strongly electron-donating ligands. The team prepared P(tmp)3 (pictured) from phosphorous trichloride and 1,2,5-trimethylpyrrole (Htmp) using triethylamine as a base, followed by the addition of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). In addition to P(tmp)3, phosphines with one or two tmp substituents were synthesized using the same protocol—for example, with chlorodiphenylphosphine or dichlorophenylphosphine as substrates.
The team found that the tmp substituent significantly enhances the electron donor capacity at the P atom. P(tmp)3, for example, is more electron-donating than tri-tert-butylphosphine with less steric bulk. The free tmp phosphines are stable in solution at ambient temperature. The researchers successfully prepared transition-metal complexes using the new ligands. The ligands could have applications, e.g., in catalysis and materials science.
- 1,2,5-Trimethylpyrrolyl Phosphines: A Class of Strongly Donating Arylphosphines,
Janina A. Werra, Klaus Wurst, Lukas B. Wilm, Pawel Löwe, Maike B. Röthel, Fabian Dielmann,
Organometallics 2023.
https://doi.org/10.1021/acs.organomet.3c00016