Gustavo Fernández, Germany, discusses a molecular design strategy using amphiphilic aza-BODIPY dyes that enables precise tuning of exciton coupling (J-type vs oblique) and nanoscale morphology

New Strategy to Guide Exciton Coupling And Nanoscale Morphology in Aqueous Environment
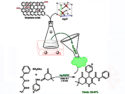
Silver–Graphene Catalyst Drives Greener Quinoline Production
A silver oxide–graphene oxide catalyst enables fast, eco-friendly synthesis of quinoline analogues with up to 97% yield in 20 minutes

Pumpkin Molecules 🎃
From molecules that give pumpkins their flavor and color to pumpkin-shaped molecules

Aqueous Circularly Polarized Luminescence Regulated by α-Helix in Homopolypeptid
Chain length and self-assembly temperature of α-helix controls aqueous circularly polarized luminescence in homopolypeptide self-assembly
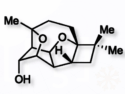
Enantioselective Total Synthesis of the Natural Product (+)-Punctaporonin U
A concise synthetic strategy combines rare cascade reactions to construct the complex, biologically active sesquiterpene (+)-punctaporonin U
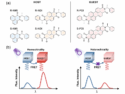
Resonance Energy Transfer Enables Chiral Recognition in the Solid State
Förster resonance energy transfer enables efficient, noncovalent chiral recognition in solid-state systems when host and guest share the same chirality

Rethinking Amines as Reactive Sites for Cross-Coupling in Drug Synthesis
A new Cu-catalyzed method converts native amines into reactive intermediates, enabling versatile cross-couplings and late-stage functionalization
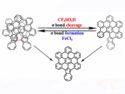
Covalent Bond Regulation in Nanographene: Precise Aggregation State Modification
Covalent bond regulation controls azulene-mediated nanographene aggregation via acids, while quenchers and temperature dictate product selectivity

High-Efficiency Photoelectrocatalytic Urea Synthesis
He Li, China, discusses using amorphous-like Cu–Zn atomic clusters on silicon nanowires to enable faster, more efficient urea formation
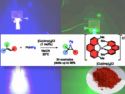
🧪 Article Highlight: Bench-Stable Copper Catalyst Simplifies Aziridination
A low-cost copper system that forms nitrogen-rich rings in minutes and serves as a light-activated tool for further transformations