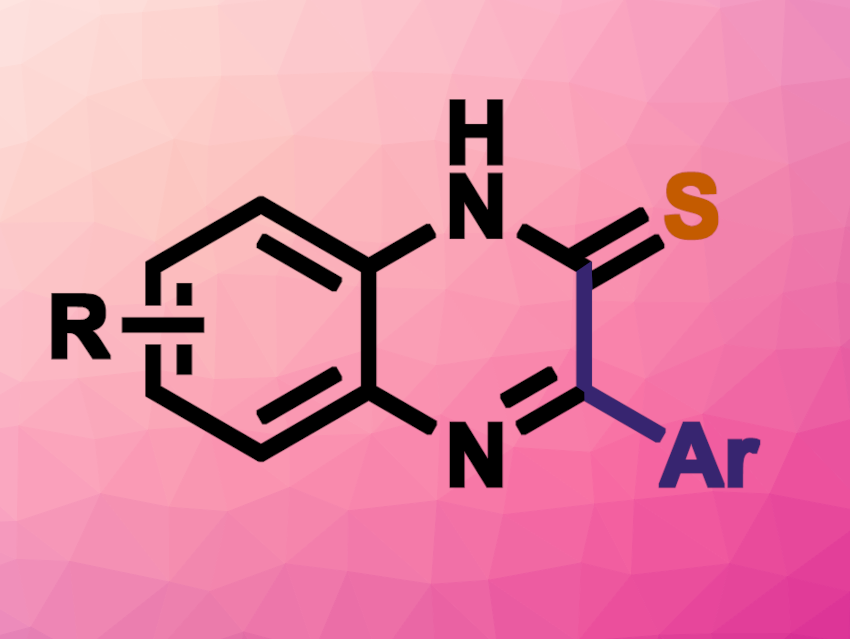
Methods for the synthesis of N-heterocycles are interesting research targets. Quinoxalines, for example, which consist of a benzene ring and a pyrazine ring, are found in bioactive compounds or functional materials. One possible synthetic path to these heterocycles is the oxidation of terminal alkynes to give 1,2-dicarbonyl compounds, followed by a condensation reaction with o-phenylenediamines. However, this generally requires transition-metal catalysts and strong oxidants.
Dinh Hung Mac, Vietnam National University, Hanoi, Thanh Binh Nguyen, CNRS, Université Paris-Sud, Université Paris-Saclay, Gif-sur-Yvette, France, and colleagues have developed a transition-metal-free method for the synthesis of quinoxaline-2-thiones using elemental sulfur. The team used DABCO (1,4-diazabicyclo[2.2. 2]octane) as a base and dimethyl sulfoxide (DMSO) as both a terminal oxidant and solvent in reactions of phenylacetylenes with elemental sulfur and o-phenylenediamines at 80 °C.
The desired quinoxaline-2-thiones were obtained in moderate to high yields. The reaction tolerates a wide range of phenylacetylenes with both electron-donating and electron-withdrawing substituents and different o-phenylenediamines. The team proposes a reaction mechanism that involves a ring opening of S8 by DABCO, followed by the addition of a polysulfide to the alkyne and fragmentation to give a thioketene. The thioketene then reacts with the o-phenylenediamine, followed by oxidation and cyclization steps to give the desired product.
- DABCO-Catalyzed DMSO-Promoted Sulfurative 1,2-Diamination of Phenylacetylenes with Elemental Sulfur and o-Phenylenediamines: Access to Quinoxaline-2-thiones,
Thi Minh Chau Tran, Nang Duy Lai, Thai Thanh Thu Bui, Dinh Hung Mac, Thi Thu Tram Nguyen, Pascal Retailleau, Thanh Binh Nguyen,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02835