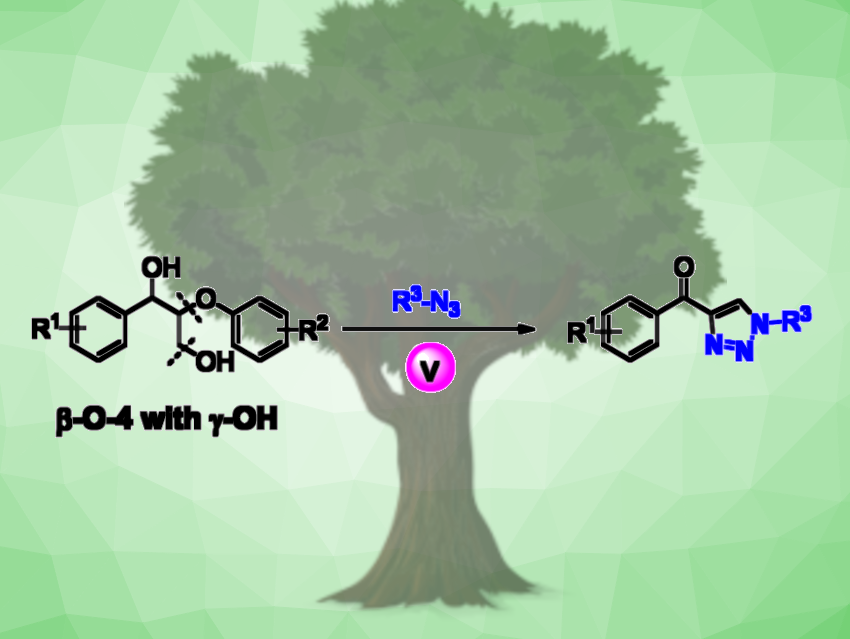
Triazoles are important heterocycles with applications, e.g., in pharmaceutical chemistry and materials science. The “traditional” way to access triazole derivatives uses click reactions between azides and alkynes. Using sustainable lignin as a starting material for triazole synthesis is interesting, but has remained challenging so far due to incompatible catalysis conditions for aryl ether bond cleavage and heterocyclic ring formation.
Tenglong Guo, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Genping Huang, Tianjin University, China, Bo Zhang, Dalian Institute of Chemical Physics and University of the Chinese Academy of Sciences, Beijing, China, and colleagues have developed a practical protocol for the synthesis of 1,2,3-triazoles, starting from lignin substructures featuring phenolic β-O-4 segments with γ-OH groups and organic azides, using a vanadium-based catalyst. The team used a catalyst that features the tridentate Schiff base (E)-2,4-di-tert-butyl-6-(((3-hydroxypropyl)imino)methyl)phenol as a ligand.
Using different azides and β-O-4-type lignin dimers, the desired 1,2,3-triazoles were obtained in mostly good to excellent yields. The team proposes that the transformation is a cascade reaction with a mechanism that includes the selective cleavage of C–O bonds, a cycloaddition, and a dehydrogenation. They propose that the vanadium-based complex acts as a bifunctional catalyst for both selective C–O bond cleavage and dehydrogenation. The developed method could be a green and sustainable way to prepare lignin-based heterocyclic aromatics, e.g., for pharmaceutical applications.
- Sustainable production of triazoles from lignin major linkages,
Wenqing Zhu, Yue Shi, Jinfei Lu, Fengan Han, Wenhao Luo, Dezhu Xu, Tenglong Guo, Genping Huang, Fritz E. Kühn, Bo Zhang, Tao Zhang,
ChemSusChem 2023.
https://doi.org/10.1002/cssc.202301421