Silaazacycles, i.e., heterocycles that contain nitrogen and silicon atoms, could be useful as alternatives to N-heterocycles in pharmaceutical chemistry. 1,4-Azasilinane derivatives, for example, have been frequently used in this context. They are six-membered rings with one N atom and one Si atom at opposite corners. 1,3-Azasilinanes are less symmetric and more challenging to synthesize, which has hampered their use in medicinal chemistry so far.
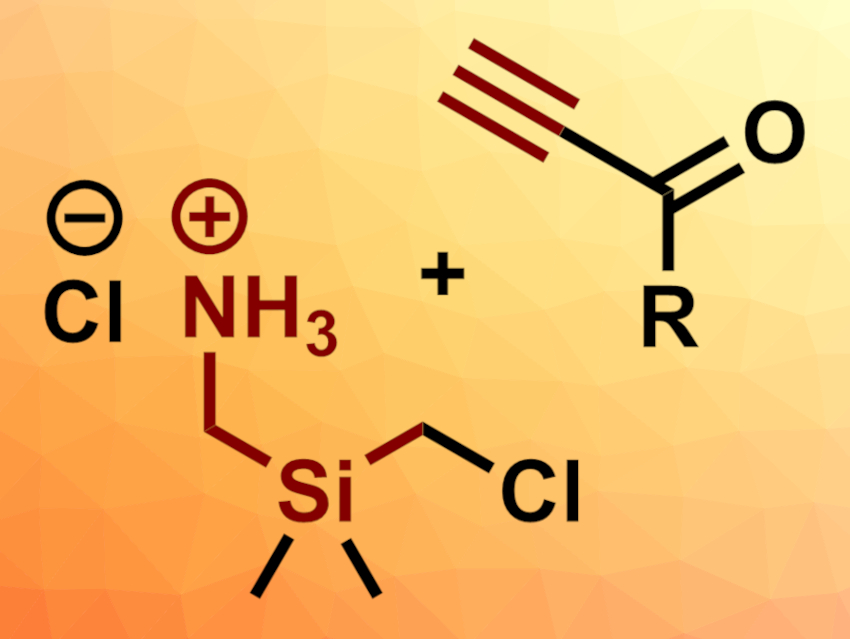
Bowen Ke, Zhenlei Song, Sichuan University, Chengdu, China, and colleagues have developed a reagent that contains N and Si and can undergo a (4 + 2) annulation with propynones (both pictured above) to give 1,3-azasilinones, a type of 1,3-azasilinane derivative. The team prepared the 1,3-N,Si reagent Cl–NH3+CH2SiMe2CH2Cl starting from Me2Si(CH2Cl)2, which was first reacted with t-BuSO2BocNH to give an N-substituted derivative. The two substituents at the nitrogen atom were then removed under acidic conditions to obtain the desired 1,3-N,Si reagent.
The developed reagent was used in (4 + 2) annulation reactions with a variety of propynones. The reactions were performed at 70 °C, using NaHCO3 as a base, MeCN as the solvent, and NaI and 4 Å MS (molecular sieve) as additives. The scope includes propynones with aryl, heterocycle, alkyl, alkenyl, and alkynyl substituents. Internal alkynes, however, were unsuitable substrates. The desired 1,3-azasilinones were obtained in generally good yields. According to the researchers, the developed reagent and similar compounds could be useful in the convenient synthesis of silaazacycles.
- (4 + 2) Annulation of Cl–NH3+CH2SiMe2CH2Cl and Propynones for the Synthesis of 1,3-Azasilinones,
Chang Ma, Yu Fan, Chunmei Zheng, Lu Gao, Wanshu Wang, Bowen Ke, Zhenlei Song,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02665