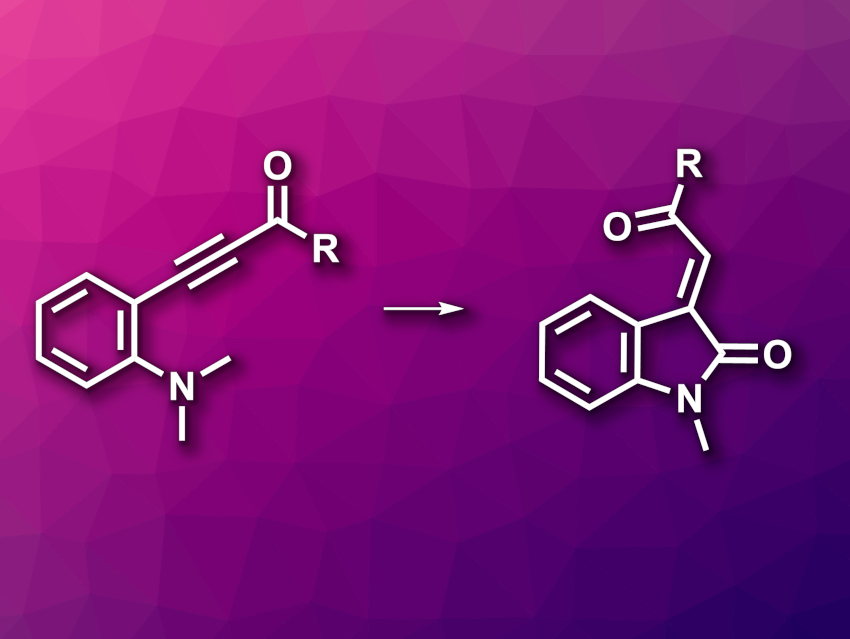
3-Alkenyl-2-oxindoles are indole derivatives. This structure is found, e.g., in natural products and bioactive compounds. They can be prepared, e.g., via condensation reactions using existing oxindoles or via transition-metal-catalyzed annulations using aniline derivatives, sometimes using toxic CO as a carbonyl source. New methods for the metal- and CO-free synthesis of 3-alkenyl-2-oxindoles would, thus, be useful.
Qiuling Song, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, China, Huaqiao University, Xiamen, China, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Henan Normal University, Xinxiang, China, and colleagues have developed a strategy for the synthesis of 3-alkenyl-2-oxindole derivatives from accessible alkynones without transition-metal catalysis (general reaction pictured). The team reacted a range of ortho-amino aryl alkynones with diethylbromodifluorophosphate, which acts as a difluorocarbene precursor in the presence of potassium carbonate as a base and oxone as an additive, using CH3CN as the solvent. The reactions were performed at 70 °C.
The desired 3-alkenyl-2-oxindole derivatives were obtained in moderate to high yields. The reaction showed a broad substrate scope and was used for the transformation of complex drug-like molecules. The team also performed the reaction on a gram scale, obtaining a yield of 78 %. According to the researchers, the difluorocarbene promotes the cleavage of C–N bonds and acts as a carbonyl C source.
- Difluorocarbene-Enabled Synthesis of 3-Alkenyl-2-oxindoles from ortho-Aminophenylacetylenes,
Shanglin Chen, Hua Huang, Xin Li, Xingxing Ma, Jianke Su, Qiuling Song,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00150