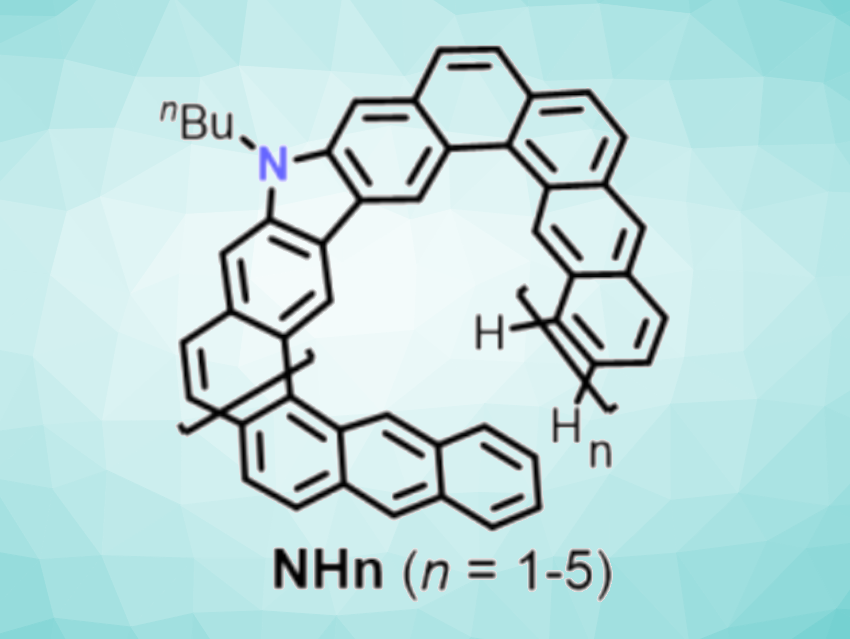
Helicenes are polycyclic aromatic hydrocarbons (PAHs) with a screw-shaped structure. They are chiral molecules that can show circular dichroism (CD) and circularly polarized luminescence (CPL) and could have applications, e.g., in asymmetric catalysis, nonlinear optics, or sensors. In expanded helicenes, the size of the molecule is increased by adding fused rings with alternating linear and angular ring fusion. They could also be interesting for their photophysical properties, but often the enantiomers are not separated because of low racemization barriers. Introducing nitrogen heteroatoms into the backbone of expanded helicenes gives expanded azahelicenes, which could have enhanced chiroptical properties.
Hai-Bo Yang, East China Normal University, Shanghai, China, Jishan Wu, National University of Singapore, and colleagues have synthesized a series of expanded azahelicenes (NHn, pictured) consisting of 11, 19, 27, 35, and 43 fused rings, respectively, and isolated the P– and M-enantiomers for three of the compounds (n = 2–4) using chiral high-performance liquid chromatography (HPLC). The team started from 3,6‐dibromo‐9‐butyl‐9H‐carbazole‐2,7‐dicarbaldehyde, which was converted to a 3,6‐dibromo‐9‐butyl‐9H‐carbazole‐2,7‐divinylether, and 1,8-dichloroanthracene, which was borylated using bis(pinacolato)diboron. Suzuki couplings between the resulting building blocks then gave oligomer precursors with n = 1–5, which were isolated using gel permeation chromatography (GPC).
The precursors were converted to the desired fused azahelicenes using a Bi(OTf)3-mediated cyclization reaction. The pure products were isolated as yellow powders using GPC with overall yields of ca. 39 %, 22 %, 11 %, 9 %, and 2 % for n = 1–5, respectively. According to the researchers, the largest example with 43 fused rings represents a new record for azahelicenes. The team found that the separated enantiomers for the compounds with n = 2–4 show excellent chiroptical properties with large absorption and luminescence dissymmetry factors.
- Expanded Azahelicenes with Large Dissymmetry Factors,
Gui-Fei Huo, Wei-Tao Xu, Yi Han, Jun Zhu, Xudong Hou, Wei Fan, Yong Ni, Shaofei Wu, Hai-Bo Yang, Jishan Wu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202403149