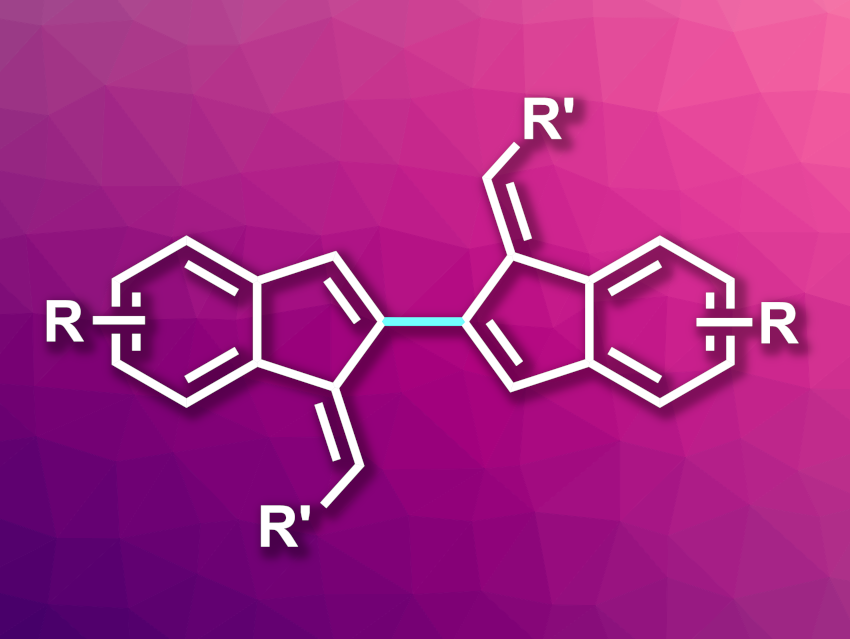
Benzofulvenes contain a benzene ring fused to a five-membered fulvene. They can be useful intermediates in organic synthesis and can serve as bioisosteres of indole units (i.e., chemically similar groups that produce similar biological properties) in pharmaceutically active molecules. Benzofulvenes can also have useful optoelectronic properties, and the electronic properties of the system can be tuned by changing the substituent on the fulvene’s exocyclic methylene group. In addition, the conjugated system of benzofulvenes could be extended via dimerization to give bis-benzofulvenes.
Venkataraman Ganeshy, Indian Institute of Technology Kharagpur, West Bengal, India, and colleagues have developed a method for the synthesis of bis-benzofulvenes. The team started from gem-dibromoolefin derivatives, which were converted to the corresponding bromobenzofulvenes using a palladium-catalyzed intramolecular Heck cyclization. The researchers used Pd(OAc)2 as the catalyst, PPh3 as a ligand, Cs2CO3 as a base, and toluene as the solvent.
The resulting bromobenzofulvene derivatives were then dimerized using a stoichiometric amount of bis(cyclooctadiene)nickel(0) (Ni(COD)2) with a bipyridine ligand in tetrahydrofuran (THF) at 65 °C. The team studied the optical and electrochemical properties of the products. They found that electron-withdrawing groups at the exocyclic methylene unit and electron-donating groups on the aromatic ring narrowed the HOMO–LUMO gap (HOMO: highest occupied molecular orbital, LUMO: lowest unoccupied molecular orbital), which enhances the optoelectronic properties of the compounds.
- Bis-benzofulvenes: Synthesis and Studies on Their Optoelectronic Properties,
Sourav Mondal, Tamal Ballav, Tofayel Sheikh Mohammad, Venkataraman Ganesh,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01318


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
