Organophosphorus compounds are used in a variety of applications, e.g., in agricultural chemistry. Methods for the introduction of phosphorus-containing groups into organic molecules are, thus, useful. This can be achieved, for example, via coupling reactions of metal phosphorus reagents and aryl halides or via radical phosphorylations. Introducing another functional group at the same time could give phosphorylation reactions even higher utility.
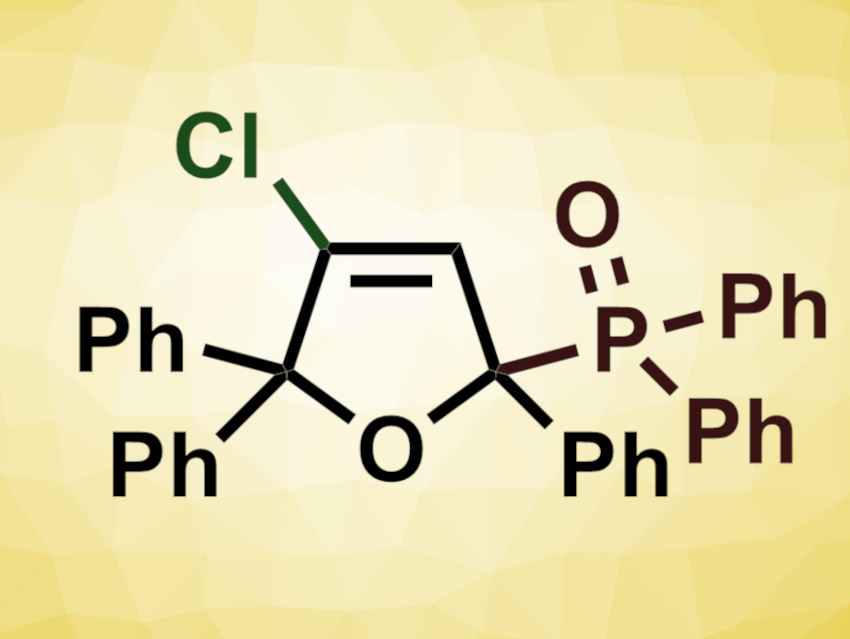
Yi-Feng Qiu, Zheng-Jun Quan, Xi-Cun Wang, Northwest Normal University, Lanzhou, China, Yong-Min Liang, Lanzhou University, China, and colleagues have developed a halophosphorylation cycloisomerization of γ-hydroxyl ynones with diphenylphosphine oxides to obtain halophosphorylated dihydrofurans (example pictured). The team reacted different substituted 4-hydroxyl-1,4,4-triphenylbut-2-yn-1-one derivatives with diphenylphosphine oxide (O=PPh2H) in the presence of trimethylsilyl chloride (TMSCl) at 65 °C.
The desired chlorophosphorylated dihydrofurans were obtained in moderate to excellent yields. The use of substituted derivatives of diphenylphosphine oxide was also possible. When the team used TMSBr instead of TMSCl, they obtained bromophosphorylated dihydrofurans in moderate to good yields. According to the researchers, the halotrimethylsilane serves as both promoter and halogenation reagent. The transformation can be performed on a gram scale. In addition, the polysubstituted products can undergo further transformations, e.g., palladium-catalyzed coupling reactions.
- Access to Polysubstituted Halophosphorylated Dihydrofurans via Halotrimethylsilane-Promoted Cascade Cyclization of γ-Hydroxyl Ynones with Diphenylphosphine Oxides,
Yi-Feng Qiu, Shi-Peng Chen, Jian-He Cao, Shutao Wang, Jin-Hao Li, Ming Li, Zheng-Jun Quan, Xi-Cun Wang, Yong-Min Liang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03323