Cyclophanes, which consist of an aromatic unit with two non-adjacent positions bridged by a chain, are interesting targets for synthesis. They can have applications in, for example, plastics and pharmaceuticals. They are also integral to many bioactive natural products. However, small cyclophanes, especially those with stacked phenyl rings and unsymmetrical bridges, can be difficult to synthesize.
Christian Hertweck, Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute and Friedrich Schiller University, Jena, Germany, and colleagues have developed a new synthetic route to strained [3.2]paracyclophanes (pictured above). The team found that UV irradiation of an aromatic carboxylic ester tethered to a toluene moiety causes an intramolecular C–C bond formation with loss of an alcohol.
Based on, e.g., radical-starter and triplet-quenching experiments, the team proposes a reaction mechanism involving an excited triplet state, hydrogen atom transfer, and cyclization of the resulting diradical (pictured below).
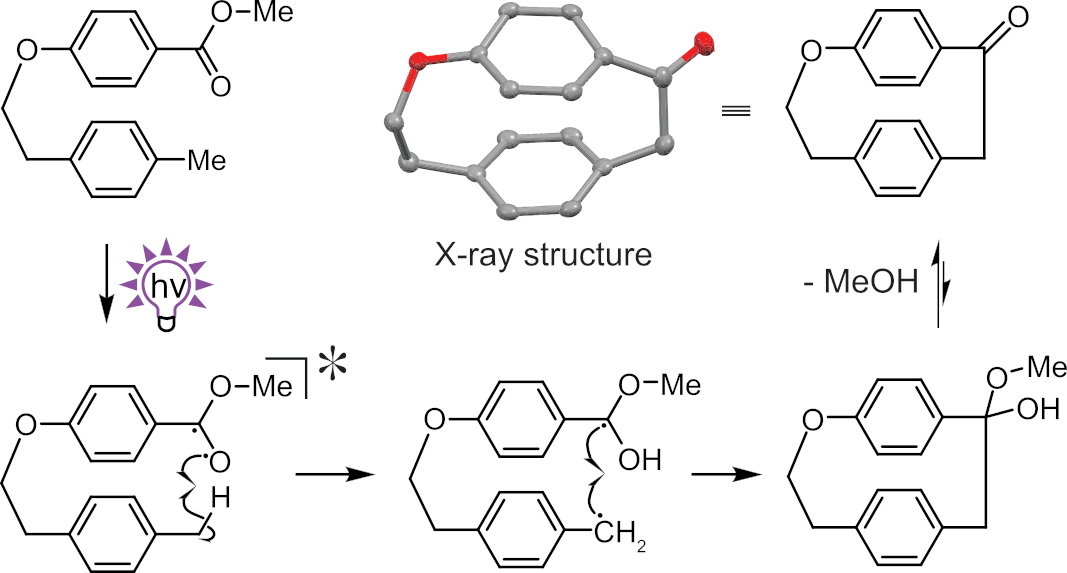
The researchers demonstrated the versatility of the photoreaction by synthesizing a range of strained paracyclophanes. The method gives good yields in a single step from readily available and varied substrates, providing a helpful addition to the synthetic toolbox. The ketone groups in the products can be useful for further functionalizations.
- A Photochemical Macrocyclization Route to Asymmetric Strained [3.2]Paracyclophanes,
Veit G. Haensch, Helmar Görls, Christian Hertweck,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202202577




