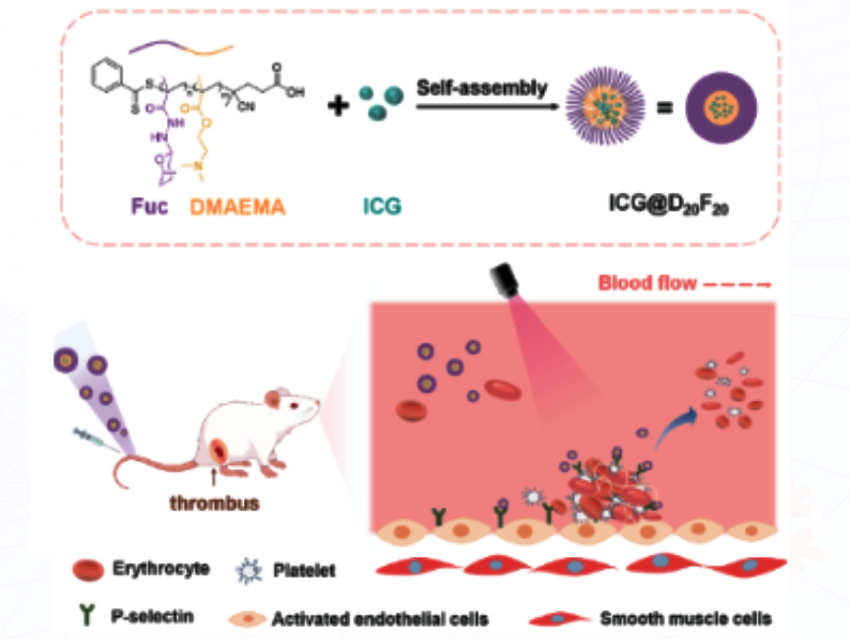
Current treatments for blood clots (thrombosis) often rely on drugs that can cause serious side effects like bleeding. There’s a need for safer, more targeted, and precise therapies that can break down clots without harming healthy tissue. This study, led by Yan Zhang, Jiangnan University, Wuxi, China, and colleagues, addresses this by developing a new biomimetic nanomaterial that can specifically target activated platelets-key components of clots, and destroy them using heat.
The researchers synthesized a polymerizable monomer, MFuc, by reacting fucose (structural component of PSGL-1 glycoprotein) with methacryloyl hydrazide under mildly acidic conditions (pH 5.5). It follows a two-step RAFT polymerization using MFuc and dimethylaminoethyl methacrylate (DMAEMA) as monomers, azobis(2-methylpropionitrile) (AIBN) as the radical initiator, and 4-cyano-4-((phenylcarbonothioyl)thio) pentanoic acid) (CTA) as the chain transfer agent. This yielded a brush-like glycopolymer, D20F20.
The resulting glycopolymers (D20F20) self-assembled into nanomicelles in water. The micelles were loaded with IR780, a dye, and thus the researchers developed ICG@D20F20, a biomimetic glycopolymer nanomicelle system. When these micelles encounter activated platelets (which express P-selectin), they bind tightly. Upon NIR irradiation (808 nm), the micelles heat up (42.1 °C at 100 µg/mL) with a conversion efficiency of 19.9% and destroy the clot through localized photothermal effects. The in vitro and in vivo tests verified effective clot targeting and breakdown.
This work presents a drug-free, targeted thrombolysis strategy that could revolutionize how blood clots are treated. It combines a hurdle technology, including biomimicry, nanotechnology, and photothermal therapy, to offer a safer alternative to conventional thrombosis treatments. Future directions include refining the micelle design, testing in larger animal models, and exploring applications in other platelet-related diseases.
- Fucose-based glycopolymeric nanomicelles for activated platelet-targeted photothermal thrombolysis
Minzhi Song, Wenxi Yang, Lingxin Peng, Changan Ren, Jinghua Chen, Yan Zhang
ChemBioChem 2025
https://doi.org/10.1002/cbic.202500253