Organoindium compounds are interesting research targets, e.g., due to their stability and tolerance against moisture. Organoindium reagents, for example, can be used as transfer reagents for alkyl, allyl, or aryl groups. In this field, comparatively little is known about perfluoroorganyl-substituted indium compounds. The electron-withdrawing nature of perfluoroalkyl substituents could make tri-substituted indanes interesting as Lewis acids, while tetra-substituted indates could be useful as weakly coordinating anions.
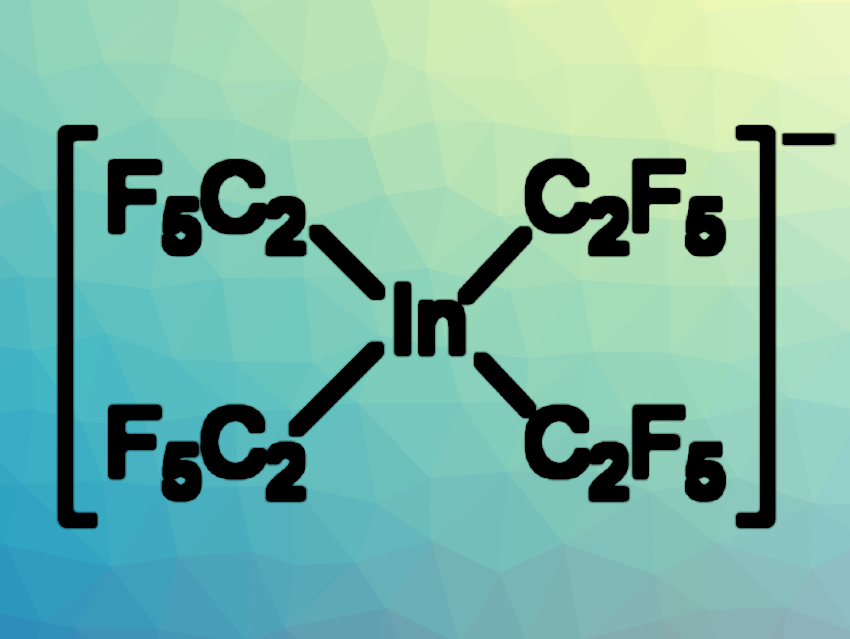
Berthold Hoge, Bielefeld University, Germany, and colleagues have investigated the influence of electron-withdrawing groups, such as perfluorinated substituents, on main group elements. The team synthesized a series of salts that contain the tetrakis(pentafluoroethyl)indate anion, [In(C2F5)4]− in combination with different cations. They first reacted indium(III) chloride with pentafluoroethyl lithium to obtain [Li(thf)n][In(C2F5)4] (thf = tetrahydrofuran).
Cation exchange reactions with with cesium fluoride or [PPh4]Cl gave Cs[In(C2F5)4] or [PPh4][In(C2F5)4], respectively. Treatment of the lithium salt with hydrochloric acid resulted in the formation of [H14O6][In(C2F5)4]2, which contains the oxonium ion [H14O6]2+. Structurally characterized oxonium ions of this composition are rare. The products were characterized using NMR and infrared (IR) spectroscopy, mass spectrometry, elemental analysis, and X-ray diffraction.
According to the researchers, the prepared tetrakis(pentafluoroethyl)indate salts represent the first fully structurally characterized perfluoroalkylindium compounds.
- Synthesis and Characterization of Tetrakis(pentafluoroethyl)indate Salts,
Sven Porath, Mira Keßler, Beate Neumann, Hans-Georg Stammler, Berthold Hoge,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202203278