Moths fear spiders for good reason—they are a spider’s favorite food, after all. Once trapped in a spiderweb, they try to escape by frantically beating their wings. However, in this situation, little ornate bella moths (Utetheisa ornatrix) remain totally calm. The spider rushes in, briefly feels its prey, and immediately cuts a hole in its web for the ornate bella moth. The moth falls out and before it hits the ground, spreads its wings and flutters away. It was certain of this outcome from the start. Its secret lies in eating the right diet from the moment it hatches, rigorous sex, and a good helping of chemistry.
1 A Small Moth in the Arctiinae Family
Almost all lifeforms—from single-celled organisms to plants, invertebrates, and primates—use chemical language to communicate, for example, in selecting mates or food, or in defending against foes. Among the nearly one million species of insects, in particular, evolution has developed amazing chemical strategies in the battle for survival, so we should be cautious in using superlatives. However, one little moth in the Arctiinae family stands out as particularly special (see Fig. 1).
 |
|
Figure 1. Utetheisa ornatrix (Dumi, wikimedia commons, CC BY-SA 3.0). |
Utetheisa ornatrix, a little moth from the wooly worm family, is native to Central America and the southern states of the USA and is distinguishable by its striking pink, white, and black wing markings. When Utetheisa ornatrix is caught in a spiderweb, even a hungry spider will cut this seemingly delicious meal out of its net, allowing the moth to fly away unscathed. (see Fig. 2).
 |
|
Figure 2. Golden silk orb-weaver spider (Nephila clavipes, Charles J. Sharp, wikimedia commons, CC BY-SA 4.0). |
2 Why Do Hungry Spiders Reject This Prey?
Thomas Eisner and his research group at Cornell University, Ithaca, NY, USA, have spent years laboriously studying this unusual behavior. They began by throwing several male and female U. ornatrix into spiderwebs. The result: Not a single one was eaten, although other moths were eaten with gusto.
As this was clearly not a matter of the spiders’ hunger, Eisner decided to do a “cross-dressing” experiment. He replaced the wings of a moth that the spiders like to eat with those of U. ornatrix. The result was surprising: After the spider had touched the wings with its legs, even the “cross-dressed” moth was cut out of the net. Because spiders can also taste with their legs it became clear that Utetheisa ornatrix tastes disgusting, at least to spiders! But why?
3 Diet and a Good Helping of Chemistry
3.1 The Rattlebox Plant (Crotalaria mucronata)
The story behind the chemistry of the ornate bella moth begins with a shrubby plant known as Crotalaria mucronata, commonly known as the rattlebox plant because its dried pods contain hard seeds that make a rattling sound when shaken (see Fig. 3).
 |
|
Figure 3. The rattlebox plant Crotalaria mucronata (Smithsonian National Museum of Natural History, CC0). |
Like all lifeforms, plants must also fight a daily battle for survival. Because they cannot run away from plant-eating animals, they would appear to be defenseless. However, if that were the case, it would be hard to explain how the Earth is still green after millions of years of insects and plants living side by side—given that most insects and many other animals devour plants all day long.
Plants just cannot be as helpless as they appear. In fact, they have evolved very highly refined chemical weapons [1]. These defensive substances are known as secondary metabolites [2] because they are not necessary for the growth and propagation of the individual plant but are an advantage in the battle for survival of the species. In contrast, no lifeform can survive without its primary metabolites, such as sugar, proteins, fats, DNA, etc.
Well over 20,000 secondary metabolites with a huge variety of structures are known. Each individual compound is only synthesized by a few species.
The chemical warfare waged by plants and fungi is primarily directed against microorganisms and insects, and is, thus, concealed from us. Sometimes, however, we are the target: Autumn crocus, death cap mushrooms, poison nuts, lily of the valley, and hemlock are not in our diet for good reason. Other secondary metabolites are quite pleasant, such as the scents of flowers or herb gardens.
3.2 Monocrotaline
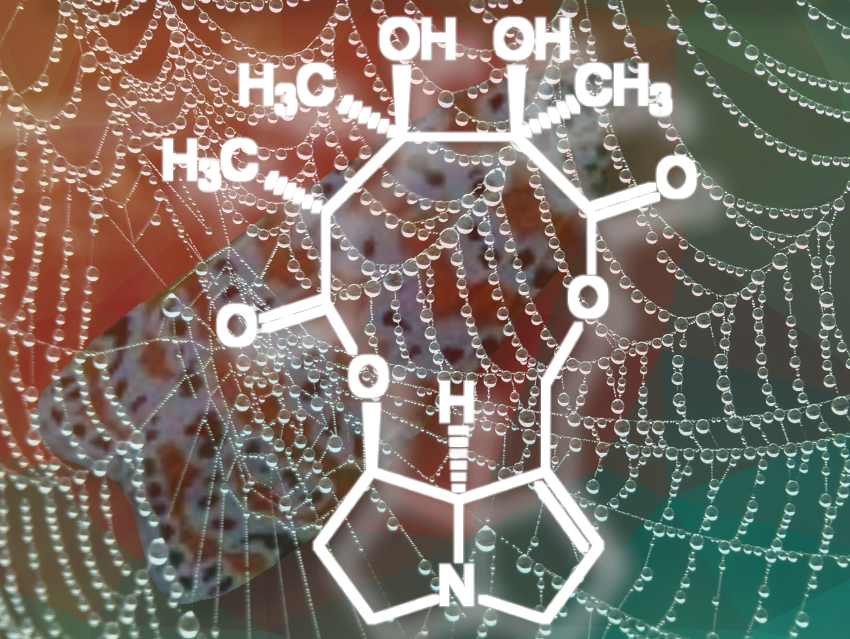
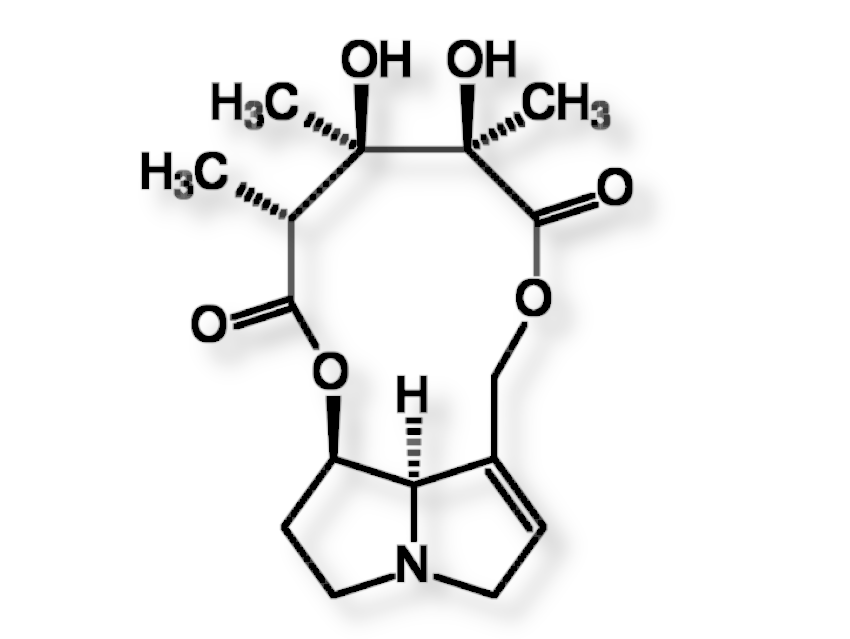
Crotalaria mucronata seems to be particularly insidious. This plant contains monocrotaline (1) (see Fig. 4), a toxin that is cytotoxic (cell damaging) and mutagenic (DNA altering) to all lifeforms, as well as hepatotoxic (liver damaging) and carcinogenic (DNA damaging) to all vertebrates (see Infobox 1).
 |
|
Figure 4. Monocrotaline (1) |
Infobox 1. Why Is Monocrotaline Carcinogenic?
(click here to expand)
The term cancer refers to a pathological malfunction of cell growth and cell division [I-1] caused by viruses, irradiation, or chemical compounds. The majority of cancers can be traced back to synthetic (e.g., smoking) or natural carcinogenic (cancer-causing) substances. Of the over 500 pyrrolizidine alkaloids occurring in Nature, only a few are carcinogenic. In these, as in monocrotaline (1), there are always esterified hydroxyl groups in the 7 and 9 positions and a double bond in the 1,2 position.
 |
|
Figure I-1. Monocrotaline (1) |
Monocrotaline is not mutagenic or carcinogenic in itself. As it is metabolized, it is dehydrated [I-2] to form dehydromonocrotaline (4) in the presence of cytochrome P450 and oxygen. Under physiological conditions, 4 hydrolyzes to form dehydroretronecine (5) within a few minutes (see Fig. I-2).
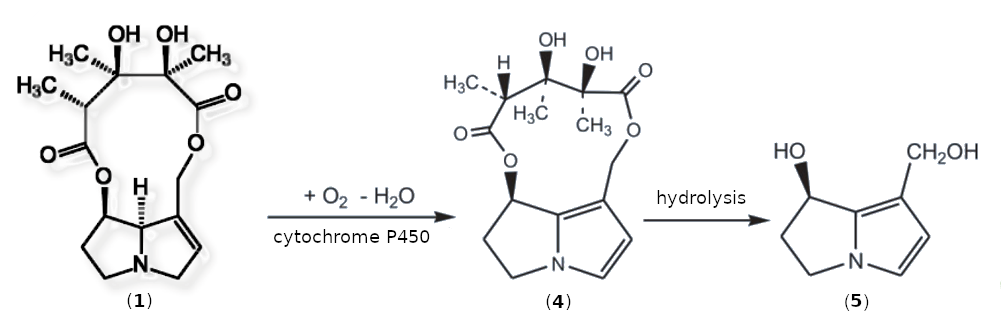 |
|
Figure I-2. Oxidative degradation of monocrotaline (1) to the carcinogenic metabolites dehydromonocrotaline (4) und dehydroretronecine (5). |
The term cytochrome P450 refers to a large group (superfamily) of enzymes that occur in nearly all organisms. The name is derived from the characteristic absorption of the carbon monoxide-cytochrome complex at 450 nm. Nonpolar foreign substances with R–H bonds are oxidized to alcohols (R–OH) with the assistance of cytochrome P450. These more polar alcohols can then be rapidly eliminated through the kidneys.
In rare cases, this detoxification sometimes backfires when substances like benzo[a]pyrene (from smoking), aflatoxin B1 (from mold), or monocrotaline are transformed into carcinogenic metabolites. In contrast to monocrotaline, both degradation products 4 and 5 are cytotoxic, hepatotoxic, mutagenic, and carcinogenic [I-3].
This comes down to chemistry: Like many other carcinogens, these two compounds are strong alkylating agents. Under physiological conditions, for example, dehydroretronecine (5) is converted to a mesomerically stabilized cation by splitting off the OH groups in the 7 and 9 positions. This cation can react with basic groups (–NH2, –OH, –SH) in proteins and DNA (see Fig. I-3).
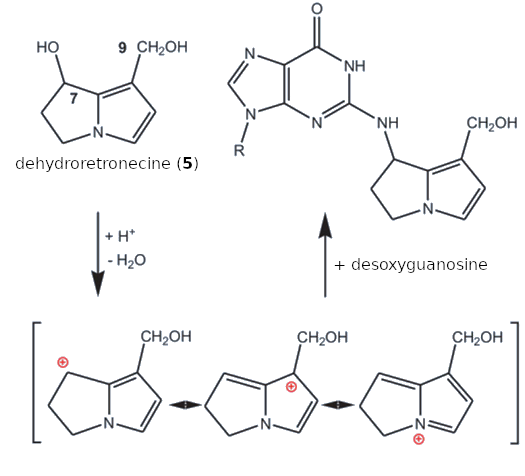 |
|
Figure I-3. Chemical origins of the carcinogenicity of dehydroretronecine (5). |
After proton acceptance and subsequent dehydration, dehydroretronecine (5) forms highly mesomerically stabilized cations that react in a conventional nucleophilic SN1 reaction with basic centers in proteins and DNA components (in this case, the guanosine group) rapidly and in an uncontrolled manner. The hydroxyl groups in both the 7 and 9 positions can react in the same way. This can also cause crosslinking between two DNA components. These are normal nucleophilic substitution reactions (SN1) at a saturated carbon atom, in which the cation that forms as an intermediate is nucleophilically attacked by the free electron pairs in the –NH2, –OR, or –SH group.
Such reactions are completely uncontrolled and have disastrous consequences. They result in severe tissue damage. Because monocrotaline is primarily oxidized in the liver, this is where damage is first noticeable in acute and chronic poisoning.
Poisoning with Alkaloid-Containing Plants
About 50 plant species contain carcinogenic pyrrolizidine alkaloids; acute and chronic poisonings have been clinically proven to result from ten plant species. Despite the bitterness of alkaloid-containing plants, they are consumed in the tropics as food or medicine. In South Africa, deaths occurred due to “bread poisoning” that was traced back to contamination of the wheat with alkaloid-containing plants.
The largest mass poisoning due to contaminated wheat occurred in northwestern Afghanistan in 1974. Almost 40,000 people were affected, 1,600 (46 % of whom were children) received clinical treatment, and many of them died within a few months.
Alkaloid-containing plants are often mixed into medicinal herbal teas, and the bitterness of the tea is mistakenly ascribed to special curative powers. Many severe poisonings in the West Indies in the 1950s were traced back to Crotalaria plant material in “bush teas”. Severe liver damage with vascular obstruction was observed in acute cases. Many of these patients later suffered from cirrhosis of the liver.
Empirical evidence indicates a causal relationship between chronic ingestion of pyrrolizidine alkaloids and liver disease and liver cancer. Despite increased education of the population in vulnerable tropical areas, roasted Crotalaria mucronata seeds are sometimes used as a substitute for coffee beans in Indonesia, for example. In exotic bush or herbal teas, poisonous plant components also find their way to central Europe and the United States, because many consciously “healthy”-eating people there blindly trust natural remedies. Crotalaria mucronata teaches us an effective lesson: Nature is not always good for us.
References
[I-1] B. Marte, Cell division and cancer, Nature 2004, 432, 293. https://doi.org/10.1038/432293a
[I-2] C. C. Yan, R. J. Huxtable, The relationship between the concentration of the pyrrolizidine alkaloid monocrotaline and the pattern of metabolites released from the isolated liver, Toxicol. Appl. Pharmacol. 1995, 130, 1–8. https://doi.org/10.1006/taap.1995.1001
[I-3] A. R. Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, London, UK, 1986.
Monocrotaline is an alkaloid (nitrogen-containing—usually botanical—compound with wide structural variation, usually classified based on its heterocyclic skeleton) with an unusual eleven-membered ring. Because of its heterocyclic skeleton, it is classified as a pyrrolizidine alkaloid [3,4]. It is primarily the bitter taste of monocrotaline that protects C. mucronate from predators. If animals eat the bitter plant despite its taste, they can only avoid acute or chronic poisoning by rapidly oxidizing monocrotaline to the non-toxic N-oxide (2) in the body (see Fig. 5 and Infobox 2).
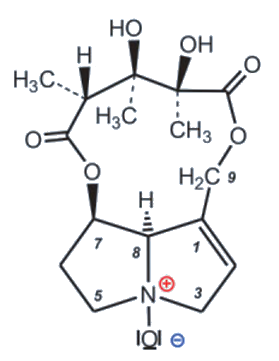 |
|
Figure 5. N-Oxide (2). |
Infobox 2. Metabolism of Monocrotaline
(click here to expand)
It is somewhat more complicated: In most plant ingredients, monocrotaline is not found as the neutral base 1, but rather as the N-oxide (2). Herbivores take up the alkaloid as the polar N-oxide, which is reduced to nonpolar monocrotaline (1) by the intestinal flora. The alkaloid can only be resorbed by the gut in its nonpolar form.
The deciding factor for toxicity is, thus, not whether the monocrotaline or its N-oxide is ingested, but whether the organism can oxidize monocrotaline to the non-toxic N-oxide within the body.
Like the carcinogenicity of monocrotaline (see Fig. 4), the “non-carcinogenicity” of the N-oxide has a chemical origin. In contrast to monocrotaline, the N-oxide cannot be dehydrated to form a stable, aromatic pyrrole system because the nitrogen atom in the N-oxide is sp3 hybridized, which prevents the formation of reactive cations.
Guinea pigs and sheep can rapidly oxidize monocrotaline (1) to the non-toxic N-oxide (2), because their liver cells contain a very effective oxygenase that catalyzes this reaction. Both of these mammals can tolerate large amounts of C. mucronata in their feed. However, rats, cattle, and humans lack this enzyme and are, thus, significantly more vulnerable.
Returning to our ornate bella moth, American chemist Jerrold Meinwald demonstrated that Utetheisa ornatrix contains 0.4 % monocrotaline, on average. This is an enormous amount. If we extrapolate that to a 70 kg human, it translates to 280 (!) g.
Where does the alkaloid come from? What does the moth use all this poison for? Why does it tolerate so much of it?
To answer these questions, we will follow the life cycle of a moth from the beginning: Female U. ornatrix lay their eggs almost exclusively on the leaves of Crotalaria mucronata. After hatching, the caterpillars initially nibble on the leaves, and when they are a little bigger, make a hole in the pods of the plant and eat the seeds for the rest of their existence as caterpillars.
The caterpillars do not just tolerate monocrotaline-containing food, they are totally crazy about it. In fact, the caterpillars have a sensitive “tongue” that can taste monocrotaline in concentrations below 10–11 mol/L, which may be a record for an insect [5]! Equipped with this highly sensitive sense of taste, it is no wonder the caterpillars can unerringly find the seeds with their extremely high monocrotaline concentrations. This exclusive diet leads to an accumulation of monocrotaline in their bodies, making them an extremely bitter and poisonous meal.
4 How Does the Caterpillar Manage Fatally High Concentrations of Monocrotaline in Its Body?
It all comes down to chemistry: Unlike other animals, the wooly bear contains an extremely effective oxygenase [6,7] that selectively catalyzes the oxidation of cytotoxic pyrrolizidine alkaloids. It, thus, converts all the monocrotaline in its body to the harmless N-oxide (see Infobox 2). The moths that emerge after pupation still contain enough monocrotaline to be protected from predators for their entire lives.
The caterpillars choose other nearby plants for pupating. This is for good reason: The high monocrotaline content of the pupae would attract foraging Utetheisa caterpillars, who would eat their own species [8]. If there is a serious shortage of monocrotaline, the larvae even eat their species’ own monocrotaline-containing eggs [9].
Thomas Eisner was able to breed U. ornatrix in the laboratory, using food with and without monocrotaline. Using these (+) and (–) animals, which could not be differentiated by their appearance, he was able to carry out a series of clever experiments.
First, both (+) and (–) moths were tossed into the webs of hungry spiders. The result was unambiguous: The spiders devoured the alkaloid-free (–) moths exclusively with no hesitation and spurned all (+) moths. This proved that the monocrotaline obtained from plants is what makes U. ornatrix unpalatable.
References
[1] D. J. Chadwick, J. A. Goode (Eds.), Insect-Plant Interactions and Induced Plant Defence, Novartis Foundation Symposium 223, John Wiley & Sons, Chichester, UK, 1999. https://doi.org/10.1002/9780470515679
[2] D. J. Chadwick, J. Whelan (Eds.), Secondary Metabolites: Their Function and Evolution, Ciba Foundation Symposium 171, John Wiley & Sons, Chichester, UK, 1992. https://doi.org/10.1002/9780470514344
[3] A. R. Mattocks, Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, London, UK, 1986.
[4] T. Hartmann, D. Ober, Biosynthesis and Metabolism of Pyrrolizidine Alkaloids in Plants and Specialized Insect Herbivores, Top. Curr. Chem. 2000, 209, 207–243. https://doi.org/10.1007/3-540-48146-X_5
[5] E. A. Bernays et al., Taste Receptors for Pyrrolizidine Alkaloids in a Monophagous Caterpillar, J. Chem. Ecology 2003, 29, 1709–1722. https://doi.org/10.1023/A:1024239201198
[6] R. Lindigkeit et al., The two Faces of Pyrrolizidine Alkaloids: the Role of the Tertiary Amine and its N-Oxide in Chemical Defense of Insects with Acquired Plant Alkaloids, Eur. J. Biochem. 1997, 245, 626–636. https://doi.org/10.1111/j.1432-1033.1997.00626.x
[7] T. Hartmann, Detoxifizierung der PAs durch spezifische N-Oxidierung, pharmbiol.phbiol.nat.tu-bs.de/ipb. (archived version accessed Dember 31, 2023)
[8] F. X. Bogner, T. Eisner, Chemical Basis of Pupal Cannibalism in a Caterpillar (Utetheisa ornatrix), Experientia 1992, 48, 97–102. https://doi.org/10.1007/BF01923618
[9] F. X. Bogner, T. Eisner, Chemical basis of egg cannibalism in a caterpillar (Utetheisa ornatrix), J. Chem. Ecol. 2001, 17, 2063–2075. https://doi.org/10.1007/BF00987992
The article has been published in German as:
- Die Chemie eines kleinen Spinners,
Klaus Roth,
Chem. unserer Zeit 2005, 39, 72–76.
https://doi.org/10.1002/ciuz.200590010
and was translated by Caroll Pohl-Ferry.
The Amazing Chemical Survival Strategy of a Moth – Part 2
Nine-hour copulation and exciting chemistry
See similar articles by Klaus Roth published on ChemistryViews.org