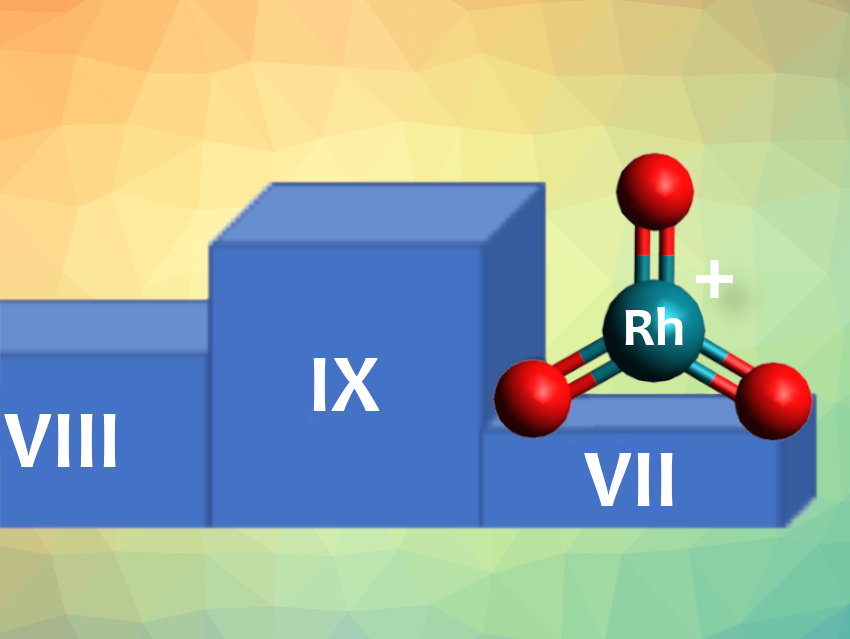
Transition metals in high or unusual oxidation states can play important roles in catalysts or reaction intermediates. However, these highly reactive species are difficult to observe in chemical reactions because they are short-lived. Examples of rhodium even in the oxidation state +6, for example, are rare.
Mayara da Silva Santos, J. Tobias Lau, University of Freiburg, Germany, and Helmholtz-Zentrum Berlin für Materialien und Energie, Germany, Sebastian Riedel, Free University Berlin, Germany, and colleagues have discovered the first rhodium species with the oxidation state +7—the third highest oxidation state of all elements. This makes rhodium the third 4d transition element to form a formal +7 oxidation state, after technetium and ruthenium.
The team identified rhodium(VII) in [RhO3]+ by isolating the species in a low-temperature ion trap in a synchrotron radiation facility. In their experiments [RhOn]+ (n = 0−3) ions were produced via argon sputtering of a rhodium target in the presence of oxygen.
The researchers used mass spectrometry (MS), X-ray absorption spectroscopy, and quantum-chemical calculations to characterize [RhO3]+. Due to its unusually high oxidation state, [RhO3]+ should be a strong oxidizing agent. According to the team, the stabilization of trioxidorhodium cations by weakly coordinating anions might be possible based on comparisons with known ions such as O2+. Understanding reaction paths that involve rhodium in high oxidation states could be useful for the design of new catalysts or improved materials.
- The Highest Oxidation State of Rhodium: Rhodium(VII) in [RhO3]+,
Mayara da Silva Santos, Tony Stüker, Max Flach, Olesya S. Ablyasova, Martin Timm, Bernd von Issendorff, Konstantin Hirsch, Vicente Zamudio-Bayer, Sebastian Riedel, J. Tobias Lau,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202207688