In Part 1, we traced the path from the licorice plant (Glycyrrhiza glabra) to the licorice wheel. Although it has proven itself over the centuries as a folk remedy for gastric disorders and diseases of the upper respiratory tract, the active ingredient in licorice, glycyrrhetinic acid, was not isolated in its pure form and characterized until 1936.
The subsequent determination of its structure was nearly a solo effort on the part of Leopold Ružička and his research group. We will accompany him on his arduous journey from the molecular formula C30H46O4 to the structural formula. In the third part of this article, we will compare how modern chemists determine the structures of such complex natural substances with the help of spectroscopic methods.
5 Chemical Structure Determination
The molecular formula of glycyrrhetinic acid, C30H46O4, was finally verified in 1936 [27]. However, the structure of the carbon framework remained fully unknown. It was only possible to verify the functional groups through chemical reactions: a carboxyl group, a secondary hydroxyl group, and an α,β-unsaturated ketone. This seems tenuous by modern standards, but we should not underestimate Ružička (see Fig. 4) and his contemporaries.
 |
|
Figure 4. Leopold Ružička (1887 – 1976). |
5.1 Double-Bond Equivalents
The team first calculated the double bond equivalent (DBE) for glycyrrhetinic acid. This parameter has fallen somewhat out of fashion but is determined by comparing the number of hydrogen atoms in glycyrrhetinic acid (C30H46O4) with those of the saturated hydrocarbon with the same number of carbon atoms (C30H62). This simple computation of the DBE is only possible for compounds containing exclusively the elements C, H, and O.
The difference of 16 hydrogen atoms in this case corresponds to a DBE of eight. The formation of a double bond or a ring formally removes two hydrogen atoms. For example, hexane is C6H14, hexene is C6H12, and cyclohexane is C6H12. For this reason, every ring formation corresponds to one DBE.
Because glycyrrhetinic acid has one carbon–carbon and two carbon–oxygen double bonds (one ketone and one carboxyl group), there are five DBE left for the C26H44 fragment: The molecular scaffold of glycyrrhetinic acid must be pentacyclic.
5.2 Dehydration Experiments
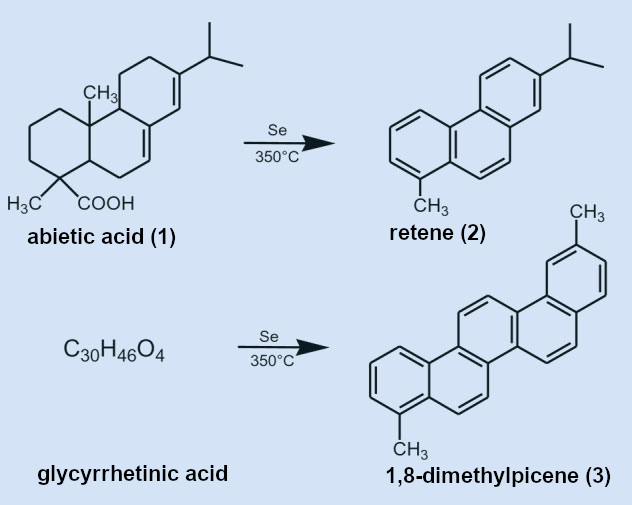
For the further elucidation of its chemical structure, glycyrrhetinic acid was heated dry (!) to 350 °C for 50 hours with elemental selenium. This dehydration procedure is nearly forgotten today but proved itself in the structural characterization of abietic acid (1) (see Fig. 5). Abietic acid is in the group of chemicals known as tricyclic diterpene acids and is a component of various conifer resins.
 |
|
Figure 5. Decomposition of abietic and glycyrrhetinic acids in the dehydration process. |
The dehydration of 1 with selenium at 350 °C causes the geminal and quaternary substituents to split off, giving the aromatic hydrocarbon retene (2). This confirmed the structure of the tricyclic abietic acid framework. The analogous reaction with glycyrrhetinic acid formed 1,8-dimethylpicene (3) (see Fig. 5). This elucidated the structure of the pentacyclic framework of glycyrrhetinic acid.
5.3 Stepwise Reduction and Dehydration Process
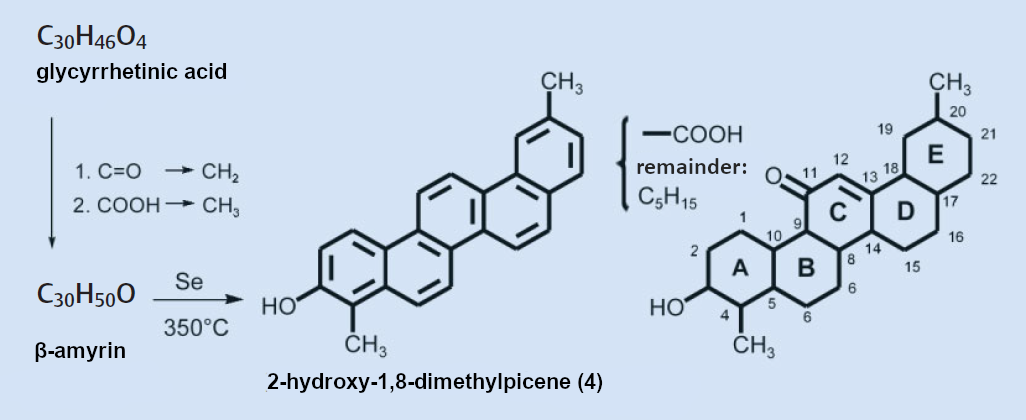
In 1939, Ružička and Marxer obtained β-amyrin through the stepwise reduction of glycyrrhetinic acid [28]. This plant substance had already been known to Ružička’s research group for years [29] (see Fig. 6). When dehydrated over selenium, β-amyrin produced 2-hydroxy-1,8-dimethylpicene (4). This established the position of the hydroxy groups of both β-amyrin and glycyrrhetinic acid as being on carbon 3. Why the dehydration of glycyrrhetinic acid does not produce any hydroxypicene remains a mystery to this day.
 |
|
Figure 6. A two-step reaction converts glycyrrhetinic acid to β-amyrin, which can be dehydrated over selenium to make 2-hydroxy-1,8-dimethylpicene. |
In addition, degradation reactions confirm a double bond between C12 and C13, making C11 the only possible position for the conjugated ketone group in glycyrrhetinic acid. Ružička speculated that glycyrrhetinic acid (C30H46O4) must be a triterpene [30] with six isoprene units.
5.4 The Isoprene Rule
Beginning in the early 1920s, Ružička and his co-workers studied terpenes, a heterogeneous class of natural products found in the flowers, leaves, needles, and resins of over 2,000 plant species. Many components of essential oils, from lily of the valley to cumin, from oranges to eucalyptus, are structural derivatives of the hydrocarbon terpene C10H16.
Ružička recognized that the basic structural unit of these substances is not terpene C10H16 but isoprene (2-methyl-1,3-butadiene, C5H8). In 1922, he formulated the isoprene rule, which declares that terpenes (as well as steroids) are formally built up of linear chains of isoprene units [31].
Let us try to follow Ružička’s thinking in formulating a hypothetical structural formula. At this point, we will simplify greatly because Ružička proposed several structural formulas for glycyrrhetinic acid over the course of his research. To learn more about the many errors and complications that occurred in the structural determination of glycyrrhetinic acid and other triterpenes, see [32].
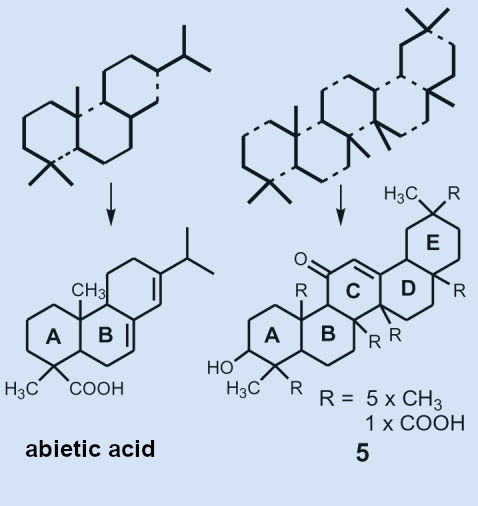
In rings A, B, and C, the carbon framework determined to that point (see Fig. 7) was closely related to abietic acid (1). Assuming that rings A and B are identical, application of the isoprene rule led to structural formula (5) (see Fig. 8).
 |
|
Figure 7. The carbon backbones of the terpenes and proposed structure 5, in which the six R fragments represent five methyl and one carboxyl group. |
The carbon backbones of the terpenes can formally be constructed by linking together isoprene units. Abietic acid is made up of four isoprene units, glycyrrhetinic acid has six (see Fig. 8). In subsequent years, Ružička and his co-workers worked intensely to either prove or disprove structural formula 5. In the end, all experiments agreed with this structure.
5.5 Position of the Carboxyl Group
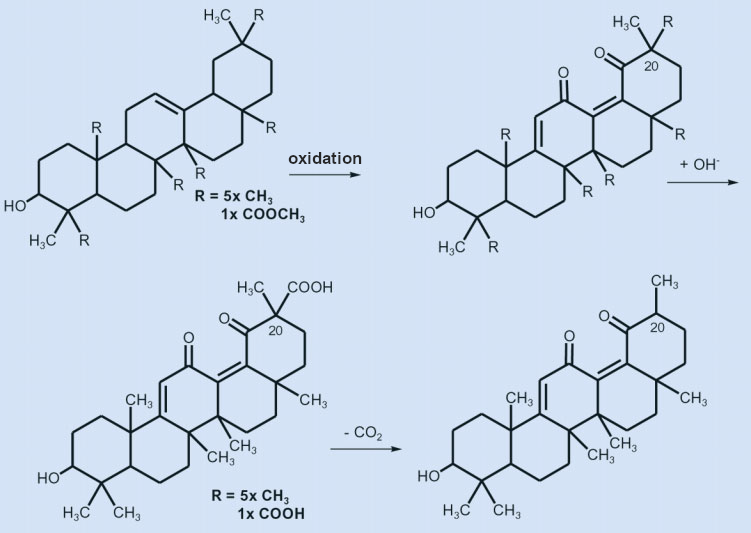
The position of the carboxyl group, however, remained uncertain. Ružička was able to carry out the deciding reaction sequence in 1943 [33]. Oxidation of the methyl ester of deoxoglycyrrhetinic acid with selenium dioxide attacked the double bond, resulting in a doubly unsaturated 1,4-diketone (see Fig. 8, left). By carrying out an alkaline saponification, Ružička hoped to obtain the free carboxylic acid from the methyl ester (see Fig. 8). However, this was unsuccessful because the resulting acid immediately split off carbon dioxide.
 |
|
Figure 8. The oxidation of the methyl ester of 11-deoxyglycyrrhetinic acid with selenium dioxide to form the diketone. |
This type of easy decarboxylation in an alkaline medium is characteristic of β-keto carboxylic acids. In an alkaline medium, the acid R-CO-CR2-COOH is converted to the anion R-CO-CR2-COO–. Cleavage of CO2 from this anion is energetically favorable because the resulting carbanion R-CO-CR2– is stabilized due to resonance with the enolate R-CO–=CR2.
This meant that the carboxyl group had to be in a β position relative to a ketone, which puts it at C20. This determined the constitution of glycyrrhetinic acid (see Fig. 9, left)!
5.6 Stereogenic Carbon Atoms
However, a complete structure determination also includes the determination of the configurations of all nine stereogenic carbon atoms, meaning the carbon atoms attached to four different substituents. This was first accomplished in 1955 after many chemical degradation reactions.
According to those results, glycyrrhetinic acid is a 3β-hydroxy-11-oxoolean-12-en-30-acid. The nine stereogenic carbon atoms have the following configurations ]: 3β, 5α [H], 8β, 9α [H], 10β, 14α, 17β, 18β [H], 30β (see Fig. 9, right).
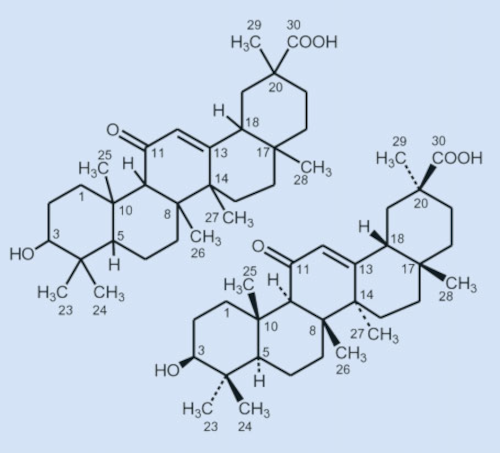 |
|
Figure 9. After years of arduous degradation and rearrangement reactions, Ružička and his group were finally able to determine the constitution of glycyrrhetinic acid in 1943 (left). |
References
[27] L. Ružička, H. Leuenberger, Polyterpene und Polyterpenoide CIX. Zur Kenntnis der Glycyrrhetinsäure, Helv. Chim. Acta 1936, 19, 1402–1406. https://doi.org/10.1002/hlca.193601901187
[28] L. Ružička, A. Marxer, Zur Kenntnis der Triterpene. (44. Mitteilung). Umwandlung der Glycyrrhetinsäure in β-Amyrin, Helv. Chim. Acta 1939, 22, 195–201. https://doi.org/10.1002/hlca.19390220125
[29] B. Becker et al. (Eds.), Fortschritte der Chemie Organischer Naturstoffe, Bd. VII, Springer Verlag, Berlin, Germany, 1950.
[30] Carl Heinz Brieskorn, Triterpenoide, physiologische Funktionen und therapeutische Eigenschaften, Pharm. unserer Zeit 1987, 16, 161–180. https://doi.org/10.1002/pauz.19870160602
[31] L. Ružička, The isoprene rule and the biogenesis of terpenic compounds, Experimentia 1953, 9, 357–367. https://doi.org/10.1007/BF02167631
[32] J. Simonsen, W. C. J. Ross (Eds.), The Terpenes, Vol. V, Cambridge University Press, UK, 1957.
[33] L. Ružička, O. Jeger, M. Winter, Zur Kenntnis der Triterpene. (75. Mitteilung). Zur Lage der Carboxylgruppe bei der Oleanolsäure und der Glycyrrhetinsäure, Helv. Chim. Acta 1943, 26, 265–279. https://doi.org/10.1002/hlca.19430260135
The article has been published in German as:
- Die Lakritzschnecke,
Klaus Roth,
Chem. unserer Zeit 2004, 38, 202–207.
https://doi.org/10.1002/ciuz.200490046
and was translated by Caroll Pohl-Ferry.
The Licorice Wheel – Part 1
The root of the licorice plant is one of the oldest known remedies – we will take a deeper look at the chemistry of this healthy treat
The Licorice Wheel – Part 3
How do chemists today determine the structure of a complex natural substance?
See similar articles by Klaus Roth published in ChemistryViews




