Coronene is a polycyclic aromatic hydrocarbon (PAH) composed of seven benzene rings fused to form a hexagon-like structure. Coronenes often have interesting optoelectronic properties. Graphene nanoribbons (GNRs), or ribbon-shaped substructures of graphene, are nanomaterials with potential uses, e.g., in organic electronics. Examples of coronene-based GNRs, however, are rare.
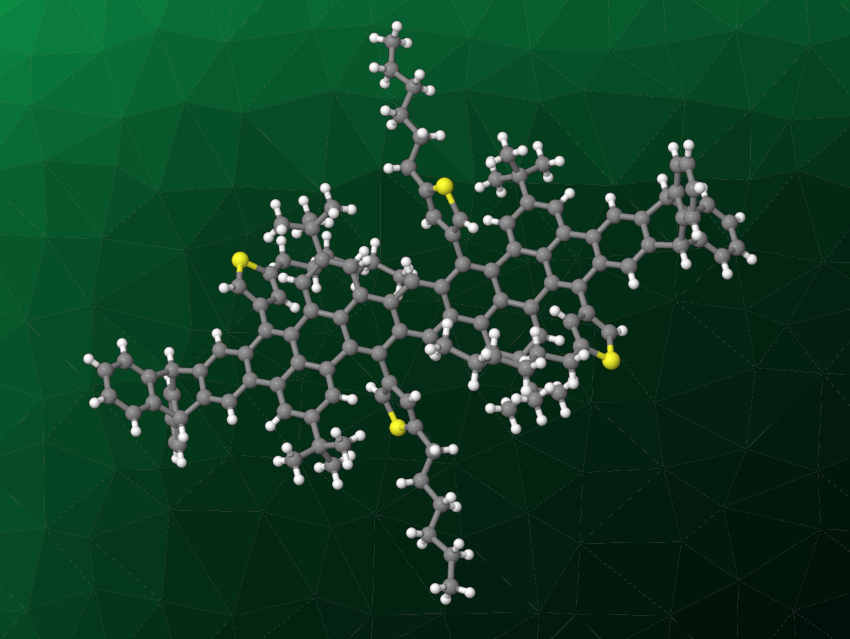
Michael Mastalerz, University of Heidelberg, Germany, and colleagues have synthesized four structurally precise thieno-fused coronene nanoribbons containing up to four coronene units, capped by triptycene ends. The team started with a bromination of triptycene end-capped perylene oligomers. The bromides were then coupled with (5-hexylthiophen-3-yl)boronic acid pinacol ester using Suzuki–Miyaura cross-coupling reactions. The resulting thienyl-substituted derivatives (example pictured) were subjected to a ring-closing reaction, i.e., an oxidative cyclodehydrogenation using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and trifluoromethanesulfonic acid. This path led to two shorter nanoribbons with one and two coronene units, respectively.
The two longer nanoribbons with three or four coronene units were prepared via iterative cross-coupling reactions using triptycene derivatives as precursors for the end-caps and a borylated dihydroanthracene and an aromatic dibromide as building blocks for the ribbon. All four nanoribbons are soluble and were fully characterized. The longest example has a conjugated backbone with a length of ca. 4.2 nm. According to the researchers, this is the first approach to the preparation of thienyl-annulated coronene nanoribbons of precise length, and the results could lead to GNRs with tunable properties for organic electronic applications.
- A Series of Soluble Thieno-Fused Coronene Nanoribbons of Precise Lengths,
Xuan Yang, Sven M. Elbert, Frank Rominger, Michael Mastalerz,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c02645