Ligand-protected metal nanoclusters represent a class of nanomaterials with atomically precise structures, composed of a finite number of metal atoms (typically several to hundreds) coordinated with surface ligands. Owing to their unique structural features and quantum confinement effects, metal nanoclusters exhibit broad application potential in fields such as luminescent materials, optoelectronic devices, and biomedicine. However, traditional synthetic strategies are limited by the complexity of nanocluster formation and their inherent instability. Consequently, achieving efficient and controllable synthesis of metal nanoclusters with excellent stability remains a key scientific challenge for advancing practical applications.
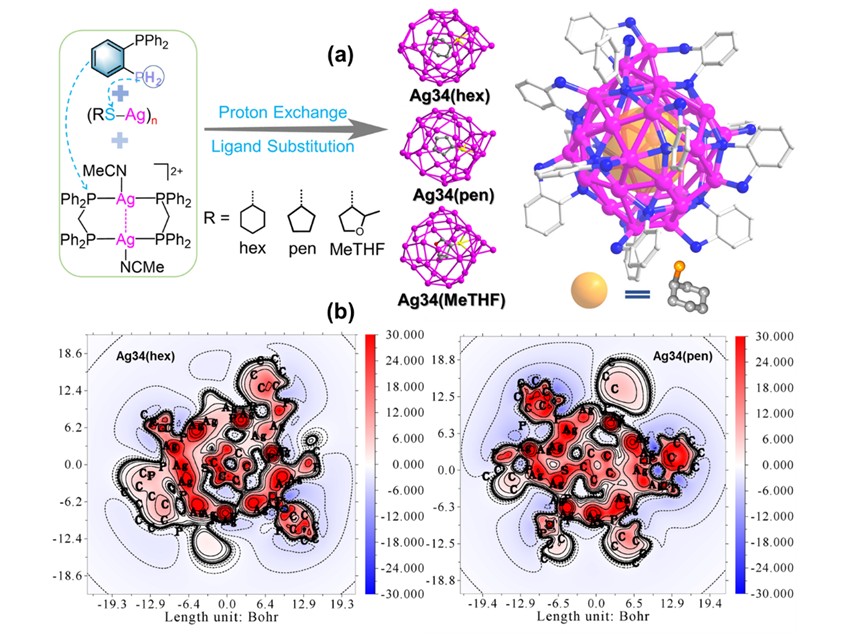
Zhong-Ning Chen, Chinese Academy of Sciences, Fujian , China, and colleagues introduced a protective ligand—primary-tertiary diphosphine ligand (2-Ph₂PC₆H₄PH₂)—and developed an “dual-exchange” synthetic strategy based on the synergistic effects of ligand exchange and proton exchange. Using this approach, three structurally similar Ag₃₄ nanoclusters were successfully prepared. Notably, in these Ag₃₄ nanoclusters, thiolate (RS⁻) defies its conventional role as merely a protective ligand and instead functions as a structural template embedded within the Ag₃₄ metal core.
The Ag₃₄ nanocluster kernel consists of 34 Ag⁺ atoms stabilized by multi-bridging coordination (μ₄/μ₅) of the deprotonated primary-tertiary diphosphine ligand (2-Ph₂PC₆H₄P₂⁻), forming stable Ag₄/Ag₅ polyhedral units. This structural feature provides a solid foundation for constructing spherical shells and significantly enhancing thermodynamic stability. The Ag₃₄ nanoclusters exhibit efficient near-infrared (NIR) luminescence in solution, with emission peaks above 800 nm and quantum yields exceeding 27%. Systematic investigations using temperature-dependent emissions, excited-state lifetimes, transient absorption spectroscopy, and oxygen sensitivity experiments confirm that the luminescence arises from phosphorescence of the triplet excited state (T₁).
Importantly, due to the highly electron-delocalized coordination character of the primary phosphine donor, the Ag₃₄ nanoclusters exhibit remarkable spherical metallic aromaticity. Theoretical calculations confirm that the metal cluster kernel displays electronic shielding effects analogous to peripheral benzene rings, demonstrating typical aromatic characteristics that significantly enhance thermodynamic stability.
Based on their excellent NIR luminescent properties and outstanding thermodynamic stability, solution-processed near-infrared organic light-emitting diodes (NIR-OLEDs) were fabricated using Ag₃₄ nanoclusters as NIR emitters. The NIR-OLEDs achieved a maximum external quantum efficiency (EQE) of 10.2%, with electroluminescence peaking at 818 nm, demonstrating superior near-infrared emission and highlighting the potential of luminescent nanoclusters in optoelectronic applications.
- Cooperative Construction of Silver(I) Nanoclusters of Primary-Tertiary Diphosphine Directed by Thiolate Templates Through Ligand Metathesis,
Xu-Yang Ding, Jing-Hao Wei, Lin-Xi Shi, Jin-Yun Wang, Han Cheng, Li-Yi Zhang, Zhong-Ning Chen
Aggregate 2025
https://doi.org/10.1002/agt2.70173