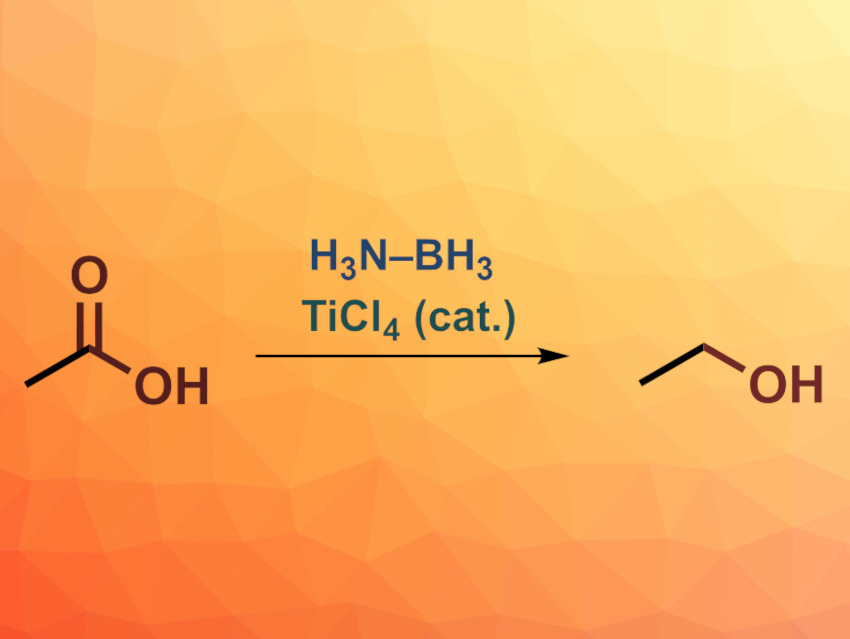
Reducing carboxylic acids directly to alcohols can be useful in organic synthesis. However, hydride reagents commonly used for this type of reaction are air- and moisture-sensitive and can suffer from a limited functional group tolerance. Other approaches also have drawbacks, e.g., a need for high pressures, high temperatures, or expensive reagents, safety issues, or esterifications as side reactions. Amine-boranes are interesting as alternative hydride reagents. They can be air- and moisture-stable, but act as weaker reducing agents.
P. Veeraraghavan Ramachandran, Purdue University, West Lafayette, IN, USA, and colleagues have developed a safe reduction protocol for carboxylic acids using H3N–BH3 and catalyzed by TiCl4, giving the corresponding alcohols as products. The team reacted a broad range of aromatic and aliphatic mono- and dicarboxylic acids with H3N–BH3 in the presence of 10 mol% TiCl4 in diethyl ether at room temperature.
The desired alcohols were obtained in mostly good to excellent yields. The reaction shows good functional group tolerance, e.g., for alkenes and nitro groups. The team also achieved selective reductions of carboxylic acids in the presence of, for example, amides or nitriles. According to the researchers, the ease of the reduction and the air- and moisture stability of the reagent could make this reaction a useful tool in organic synthesis.
- A Safer Reduction of Carboxylic Acids with Titanium Catalysis,
P. Veeraraghavan Ramachandran, Abdulkhaliq A. Alawaed, Henry J. Hamann,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03326