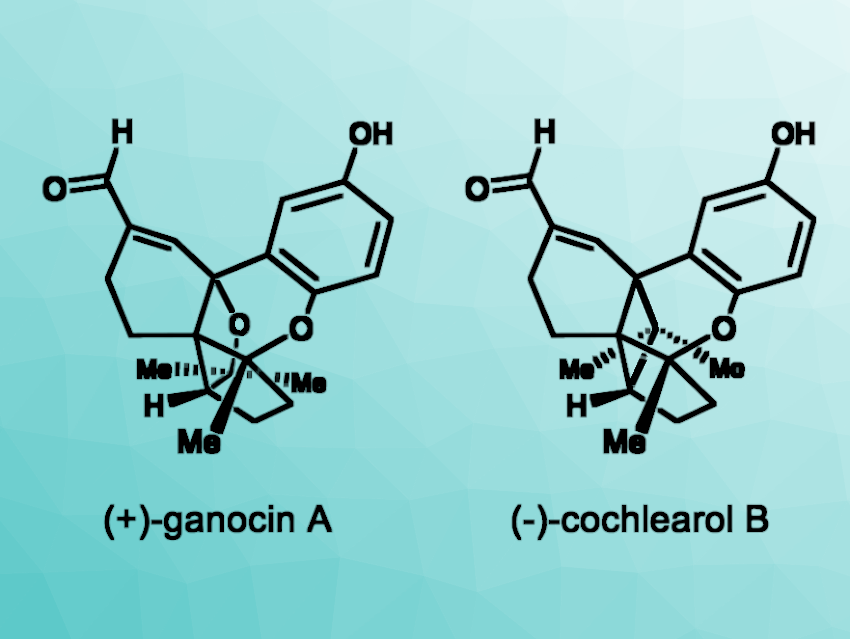
Wood-decaying fungi of the Ganoderma genus are often used in traditional Asian medicine. Natural products such as ganocin A and cochlearol B (pictured) have been isolated from Ganoderma cochlear fungi as racemates. These compounds show interesting biological activities that differ based on the respective enantiomer. Thus, approaches to the enantioselective syntheses of the compounds would be useful.
Kazuyuki Sugita, Hoshi University, Tokyo, Japan, and colleagues have performed enantioselective total syntheses of (+)-ganocin A and (–)-cochlearol B. The team started from commercially available 1-benzyloxy-3-iodobenzene, which was converted to a diketone intermediate in four steps. This was followed by an enantioselective Corey–Bakshi–Shibata reduction, the installation of a methyl group using MeMgBr, a debenzylation, and a Swern oxidation to give an enone intermediate. This intermediate was subjected to a phenolic oxidative cyclization to obtain a quinone hemiacetal.
The team used an intramolecular radical cyclization and a benzylic oxidative cyclization to construct the targeted pentacyclic skeleton. From there, the introduction of an α,β-unsaturated aldehyde and a deprotection step gave (+)-ganocin A. The common quinone hemiacetal intermediate was subjected to an intramolecular [2 + 2] photocycloaddition, followed by further transformations to give (–)-cochlearol B.
In summary, enantioselective total syntheses of (+)-ganocin A and (–)-cochlearol B were achieved in 17 steps (longest linear sequence ) and 9 % overall yield and in 16 steps (longest linear sequence) and 9 % overall yield from 1-benzyloxy-3-iodobenzene, respectively. The obtained optically active forms of both compounds could be useful in medicinal chemistry studies.
- Enantioselective Total Syntheses of (+)-Ganocin A and (−)-Cochlearol B,
Tomoya Mashiko, Yuta Shingai, Jun Sakai, Shinya Adachi, Akinobu Matsuzawa, Shogo Kamo, Kazuyuki Sugita,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03572