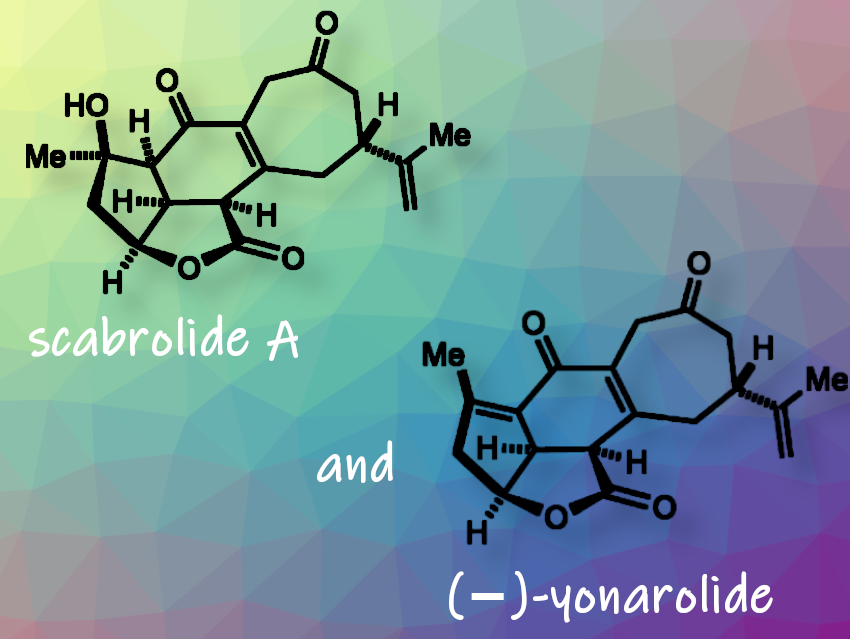
(−)-Yonarolide and (−)-scabrolide A are representative members of a class of norcembranoid diterpenoids first isolated in 1995 from the soft coral Sinularia. Although their isolation yields were low, initial studies have suggested that (-)-scabrolide A has anti-inflammatory and anticancer properties. Several similar compounds have also demonstrated cytotoxic effects. These compounds also have a unique all-cis stereochemical arrangement surrounding the central cyclohexene core and a rich oxidative decoration of the carbon framework, making them structurally noteworthy.
David Sarlah and colleagues, University of Illinois, Urbana, Il, USA, have developed an efficient total synthesis of these oxidized norcembranoid terpenoids. This was achieved in 10 and 11 steps, representing the longest linear sequence, starting from an enone obtained from (R)-linalool. The researchers constructed the carbocyclic skeleton using two chiral-pool-derived fragments. They used a [5,5]-bicyclic lactone, which was accessed through a Ni-catalyzed pentannulation of functionalized cyclopentenone with methylene cyclopropane. This method allowed for the establishment of two key stereocenters in a single operation. This innovative approach has not been previously used in total synthesis. It is analogous to the classical trimethylenemethane (TMM)-cycloaddition. However, the synergy between a Lewis acid and a nickel catalyst achieved orthogonal regio- and diastereoselectivity.
The method also incorporated other notable features, including a Liebeskind-Srogl coupling, the induction of a cyclization/elimination cascade through a zinc-amido base, and the installation of a sensitive enedione motif via late-stage γ-oxidation. They obtained (−)-yonarolide in a yield of 66 % by treating (−)-scabrolide A with Burgess reagent. The researchers anticipate that their approach will facilitate future synthetic efforts aimed at the remaining members of the norcembranoids class of natural products.
- Total Syntheses of Scabrolide A and Yonarolide,
Roberto Serrano, Yaroslav D. Boyko, Lucas W. Hernandez, Aleksandras Lotuzas, David Sarlah,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02317