The cylindricines are a family of eleven marine alkaloids isolated from the sea squirt Clavelina cylindrica. They have pyrrolo[1,2-j]quinoline or pyrido[2,1-j]quinoline azatricyclic frameworks. Cylindricines are interesting targets for synthesis. However, no total synthesis of cylindricines F–K had been reported so far, and existing enantioselective syntheses focused only on cylindricine C, which can be converted into cylindricines D and E.
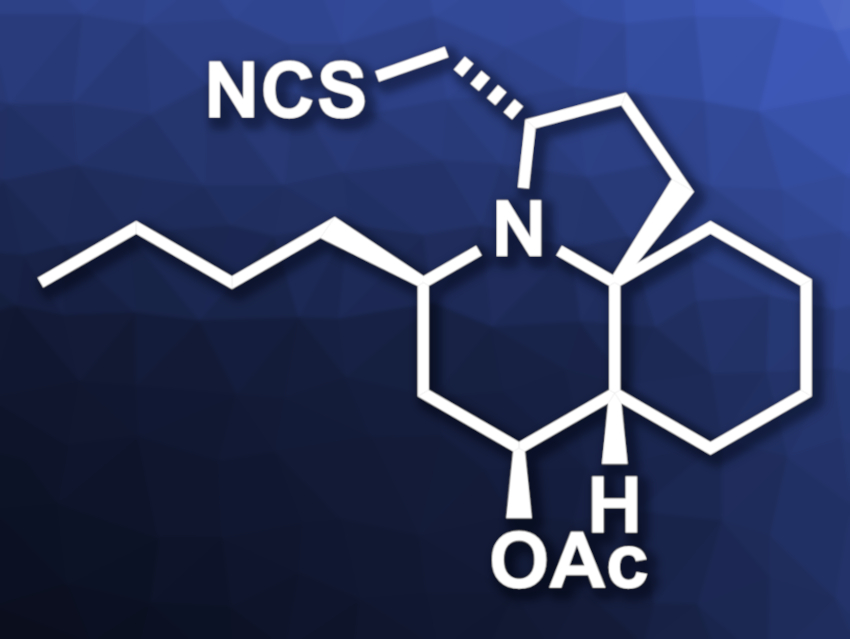
Mercedes Amat, University of Barcelona, Spain, and colleagues have performed the first total synthesis of (−)-cylindricine H (pictured). The synthesis is based on chiral aminoalcohol-derived oxazoloquinolone tricyclic lactams, which can be transformed into different substituted cis-decahydroquinoline scaffolds.
The team started from an( R)-phenylglycinol-derived tricyclic lactam. They first selectively reduced the carbonyl unit of the lactam and then installed the quarternary stereocenter at C10 of the target product via an allylation. The oxy functionality at C4 was introduced next. For this, the team used a conjugate addition of bis(pinacolato)diboron followed by oxidation to an alcohol and protection of the group. Then the butyl substituent at C2 was added in a stereoselective manner.
The five-membered pyrrolidine ring was closed by introducing an epoxide group, followed by a regioselective intramolecular ring opening of the epoxide. Finally, the SCN substituent was installed by a reaction with NH4SCN to obtain (−)-cylindricine H. According to the researchers, the synthesis shows the potential of phenylglycinol-derived tricyclic lactams for the preparation of natural products with a decahydroquinoline core.
- Total Synthesis of (−)-Cylindricine H,
Miriam Piccichè, Alexandre Pinto, Rosa Griera, Joan Bosch, Mercedes Amat,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02004