Quorum sensing (QS) is a chemical communication process between bacteria. Inhibitors of QS could prevent bacteria from causing disease without directly affecting bacterial growth, e.g., by influencing the expression of virulence factors and biofilm formation. Therefore, QS inhibitors are considered less likely to induce resistance and could serve as promising leads for the development of a new type of antibacterial agents.
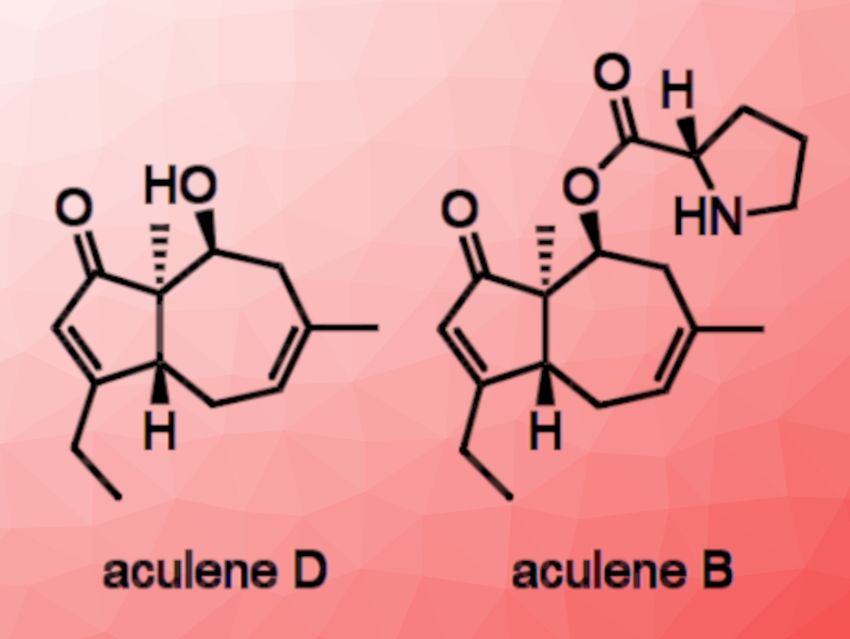
Masaru Enomoto, Tohoku University, Japan, and colleagues have performed the first total synthesis of aculenes B and D (pictured above). Aculene D exhibits QS inhibitory activity against the bacterium Chromobacterium violaceum CV026.
A key feature of the syntheses is the diastereoselective nucleophilic addition of methallylzinc bromide to an acetal-protected aldehydic intermediate to give the desired homoallylic alcohol (pictured below). This reaction showed a reversal of diastereoselectivity compared with the addition of the zinc reagent to the corresponding unprotected β-keto aldehyde, which was originally planned by the team.
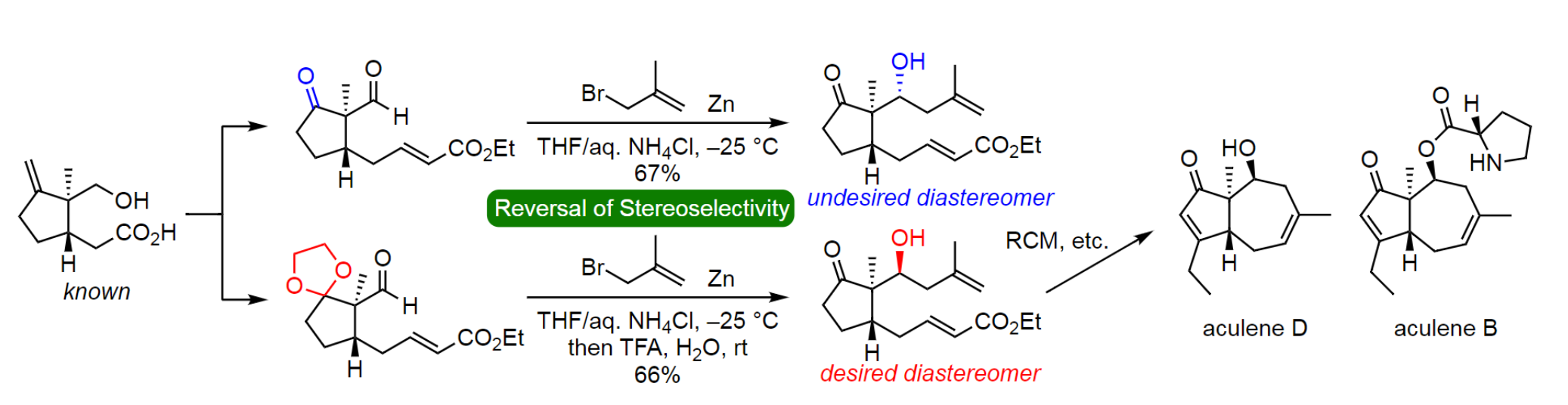
The seven-membered ring was constructed using ring-closing metathesis. An additional five-step process, including the installation of an ethyl group on the five-membered ring, was then used to convert the cyclization product to aculene D. The esterification of aculene D with an N-protected L-proline, followed by deprotection, allowed the team to also achieve the first total synthesis of aculene B. This work could also be useful for the synthesis of other natural products in the aculene family.
- Total Synthesis of Aculenes B and D,
Hinata Yokokawa, Seiya Ishizawa, Katsuya Saito, Yasuhiro Meguro, Shigefumi Kuwahara, Masaru Enomoto,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202201482