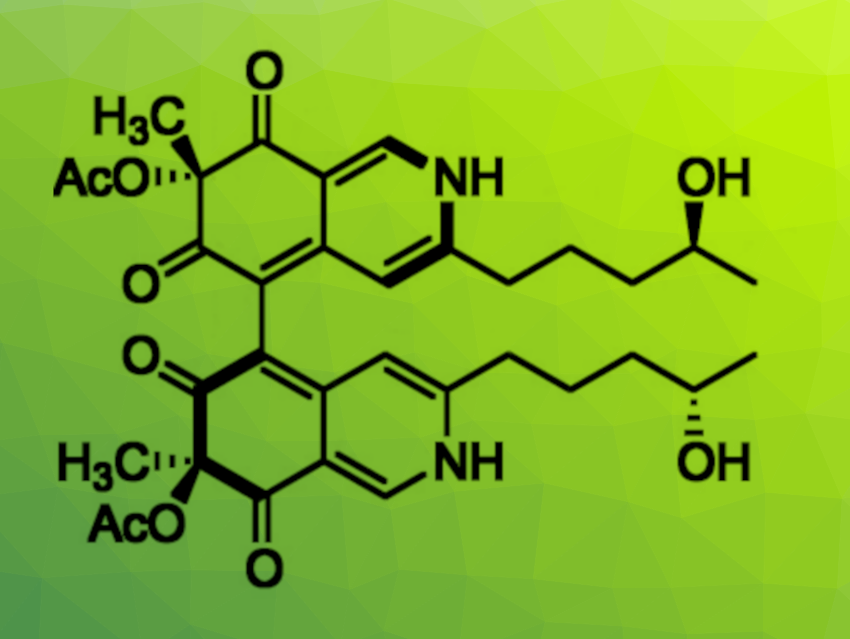
Azaphilone alkaloids are natural products with interesting biological effects and broad structural diversity. They feature highly oxygenated bicyclic cores. Chaetoglobin A (pictured), for example, is a dimeric azaphilone alkaloid that features a chiral axis connecting two azaphilones. It has shown, e.g., anticancer activities.
Houng Kang, University of Pennsylvania, Philadelphia, USA, and Chungbuk National University, Cheongju, South Korea, Marisa C. Kozlowski, University of Pennsylvania, and colleagues have performed an asymmetric total synthesis of chaetoglobin A. The team started with the synthesis of a monomer that can undergo an oxidative phenol coupling to form a dimeric intermediate. The monomer was prepared starting from a iodophenol and an acetyl-protected chiral alkyne fragment using a Sonogashira coupling. The diastereoselective oxidative phenol coupling was then performed using a chiral vanadium catalyst.
This dimerization was followed by a double formylation using a preformed Vilsmeier reagent. The resulting intermediate was then subjected to an oxidative dearomatization/protection/deprotection sequence. Finally, an amination using NH4OAc gave the desired chaetoglobin A. The NMR spectral data of the synthesized material matched that of the natural product.
- Asymmetric Total Synthesis of Chaetoglobin A,
Houng Kang, Carilyn Torruellas, Marisa C. Kozlowski,
J. Org. Chem. 2023.
https://doi.org/10.1021/acs.joc.3c00002