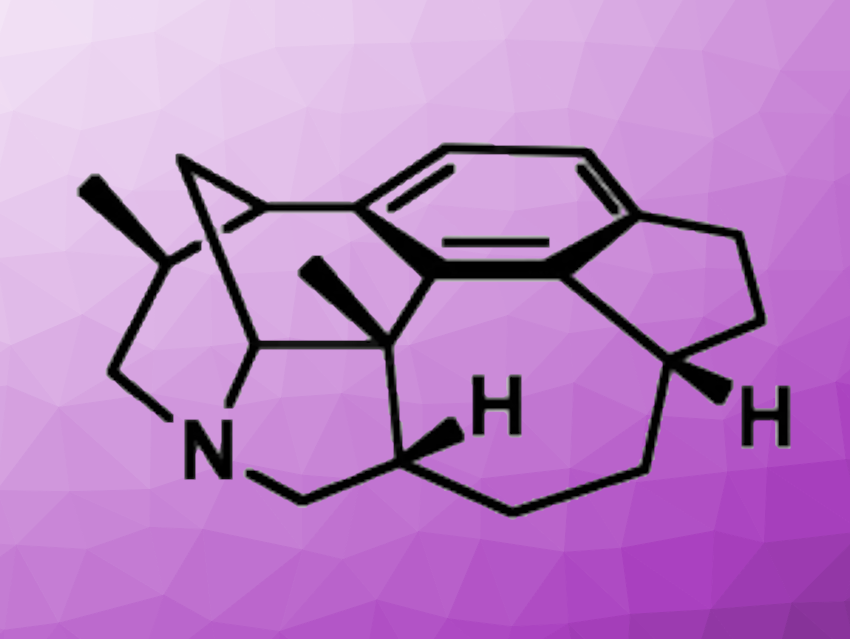
The Daphniphyllum alkaloids are polycyclic natural products first isolated from a deciduous tree. Daphenylline (pictured) is a hexacyclic Daphniphyllum alkaloid with a sterically crowded tetrasubstituted benzene ring. The compound is an interesting target for total synthesis. Existing approaches for the total synthesis of daphenylline depend on starting with the installation of one chiral center, whereas strategies that start with two stereocenters had not been used so far.
Yu-Rong Yang, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China, and colleagues have performed a concise enantioselective total synthesis of (−)-daphenylline that begins with two chiral stereocenters, C2 and C18, which are installed in one step. The team started from phenyl vinyl carbinol and propionaldehyde, which were reacted in an Ir/amine dual-catalyzed allylation to obtain a chiral aldehyde with the desired stereochemistry at the C2 and C18 centers. The aldehyde intermediate was converted into a tricyclic allylic amine in six steps: a reductive amination, a hydrocarboxylation, an intramolecular Friedel–Crafts acylation, an intramolecular amination, and a Wittig olefination.
The tricyclic allylic amine was used in an amide coupling with a known alkynyl carboxylic acid, followed by a hydrostannylation/iododestannylation to give a vinyl iodide. A Heck cyclization then gave a tetracyclic intermediate, and an oxidation step was used to install a carboxylic acid. An intramolecular Friedel–Crafts acylation was used to close the fifth ring, followed by a Wittig olefination and a hydrocarboxylation to introduce another carboxylic acid group. A third intramolecular Friedel–Crafts acylation then gave a hexacyclic ketone, which underwent deoxygenation to give the desired (−)-daphenylline.
Overall, (−)-daphenylline was synthesized in 14 steps from two commercially available starting materials. According to the researchers, the employed strategies could also be useful for other alkaloid syntheses.
- Enantioselective Total Synthesis of (−)-Daphenylline,
Bing-Lu Wu, Jian-Neng Yao, Xiang-Xi Long, Zong-Qin Tan, Xiao Liang, Li Feng, Kun Wei, Yu-Rong Yang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12741