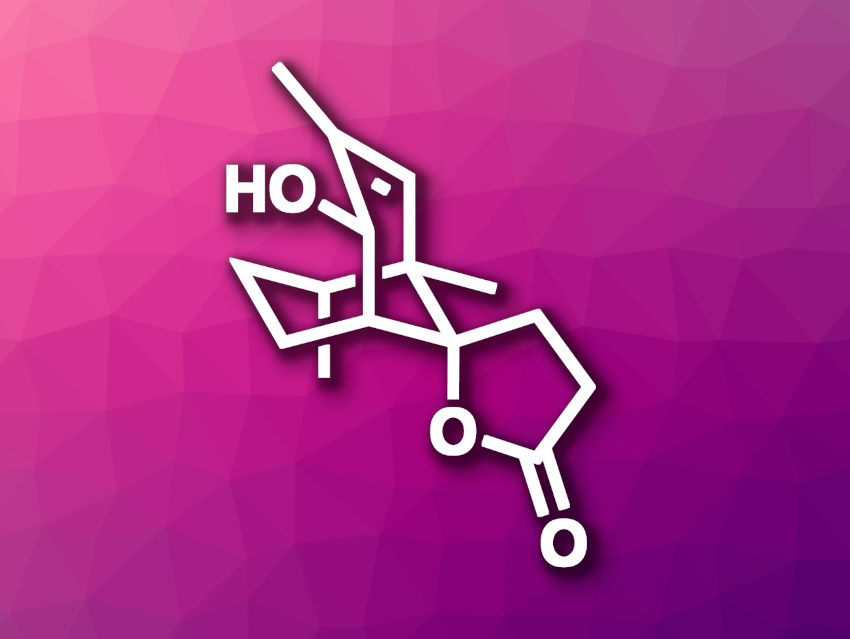
Lemnalemnane A (pictured) is a sesquiterpenoid that was first isolated from soft corals. It has an interesting structure with a spirobicyclo[3.3.1]nonanefuran core and has shown anti-inflammatory activity. So far, there had been no synthetic studies of lemnalemnane A.
Hisanaka Ito, Tokyo University of Pharmacy and Life Sciences, Japan, and colleagues have performed the first asymmetric total synthesis of lemnalemnane A. The team started from (S)-carvone, which was first converted to a diketone intermediate in a reaction sequence that involves a Dieckmann condensation. The resulting intermediate has the desired bicyclo[3.3.1]nonane skeleton. Next, a chemo- and stereoselective reduction with L-Selectride was used to introduce the OH group of the target compound.
To introduce the furan subunit, the team first used a reaction with a protected propargyl alcohol, followed by deprotection, hydrogenation of the alkyne group, and an oxidation to create the spirolactone unit via a hemiacetal intermediate. The resulting unsaturated lactone was reduced using sodium borohydride and nickel(II) chloride to obtain lemnalemnane A. Overall, (−)-lemnalemnane A was obtained in 13 steps from (S)-carvone. According to the researchers, the methodology could be extended to the synthesis of other structurally related terpenoids.
- Asymmetric Total Synthesis of (−)-Lemnalemnane A,
Toyoharu Kobayashi, Rina Sugitate, Kyohei Uchida, Yuichiro Kawamoto, Hisanaka Ito,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.3c04314