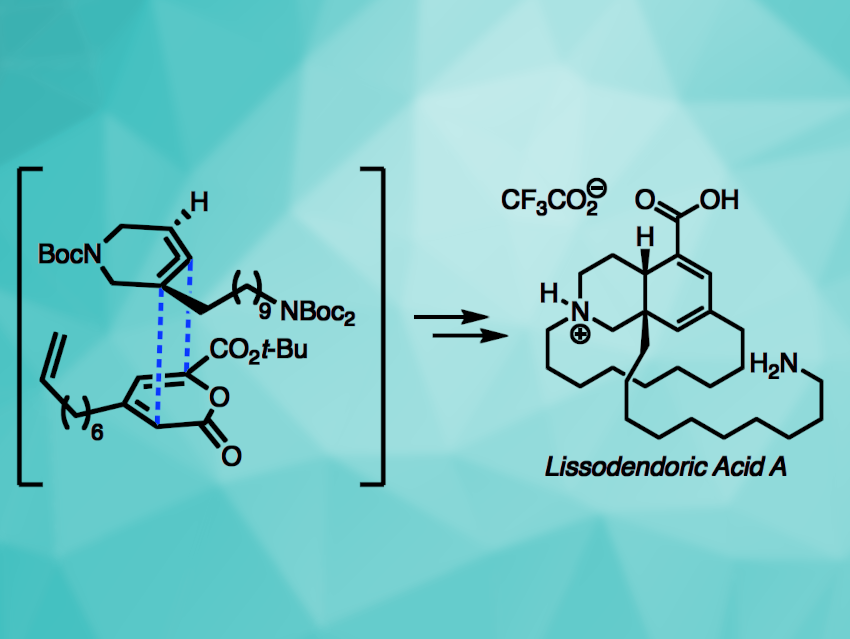
Lissodendoric acid A (pictured) is a natural product with an azadecalin core, a 14-membered macrocycle, and two stereocenters (one quaternary). Its structure poses interesting challenges for total synthesis. Strained cyclic compounds such as arynes or cyclic alkynes can be useful to drive reactions in organic synthesis. Cyclic allenes are less well-studied as strained intermediates.
Neil K. Garg, University of California, Los Angeles, USA, and colleagues have performed the first total synthesis of lissodendoric acid A, employing a Diels–Alder cycloaddition involving a strained azacyclic allene (pictured). The team started with the synthesis of a pyrone building block. A silyl triflate was then prepared to act as a precursor for an azacyclic allene, which was reacted with the pyrone in a Diels–Alder cycloaddition to build the azadecalin core. The product was further functionalized to obtain a suitable precursor for macrocyclization via ring-closing metathesis. After introducing the desired macrocycle, multiple reductions and deprotection gave the desired product.
Overall, the team obtained lissodendoric acid A in an enantioselective manner and confirmed the proposed absolute stereochemistry of the natural product. The work shows that strained cyclic allenes can be useful tools in organic synthesis, e.g., for the rapid construction of complex scaffolds.
- Total Synthesis of Lissodendoric Acid A,
Francesca M. Ippoliti, Laura G. Wonilowicz, Nathan J. Adamson, Evan R. Darzi, Joyann S. Donaldson, Daniel J. Nasrallah, Milauni M. Mehta, Andrew V. Kelleghan, K. N. Houk, Neil K. Garg,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202406676