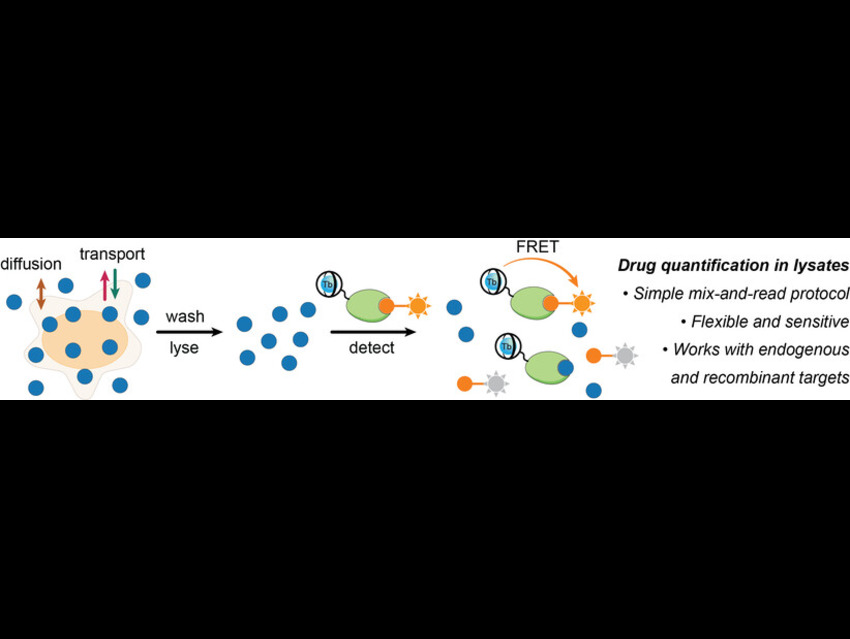
Quantifying small-molecule drugs in complex biological samples like cell lysates is challenging due to interference from proteins and other cellular components. This study addresses the need for a simple, sensitive, and high-throughput method to measure drug concentrations directly in cell lysates without extensive purification.
Researchers led by Ralph Mazitschek, Massachusetts General Hospital, Boston, USA, and colleague have developed a time-resolved Förster resonance energy transfer (TR-FRET) platform that simplifies how to measure small-molecule drugs in complex samples like cell lysates.
Traditional methods often struggle with background noise and require purification steps, but this TR-FRET system offers a modular, adaptable design that works with various drugs and delivers fast, sensitive, and accurate results, right within the messy cellular environment. The research mechanism lies in energy transfer between two fluorophores, a donor and an acceptor. In this setup, a terbium-labelled antibody acts as the donor, binding to a drug-specific tracer, while the acceptor is a dye-labelled version of the drug. When the drug present in the cell lysate competes with the labelled drug for antibody binding, the FRET signal changes proportionally, enabling accurate quantification. Thus, this shifts the signal in measurable ways, allowing researchers to determine drug levels efficiently, without washing or separation steps.
The assay uses materials such as labelled antibodies, dye-tagged drugs, and a microplate reader capable of time-resolved fluorescence detection. It is performed in a homogeneous format with no washing steps, making the process fast and scalable. Time-resolved detection further minimizes background fluorescence from the lysate, allowing sensitive and reliable drug measurement directly in complex biological samples.
This technique could transform high-throughput screening, pharmacokinetic studies, and personalized medicine by making drug analysis more accessible and scalable. Future studies may include adapting the system for multiplexed drug detection and expanding its use to multiple drug targets or other biomolecules, potentially accelerating the pace of biomedical research.
Reference:
A Time-Resolved Förster Resonance Energy Transfer Platform for the Facile Quantification of Small Molecule Drugs in Cell Lysate,
N. Connor Payne, Ralph Mazitschek,
Anal. Sens. 2025.
https://doi.org/10.1002/anse.202500047