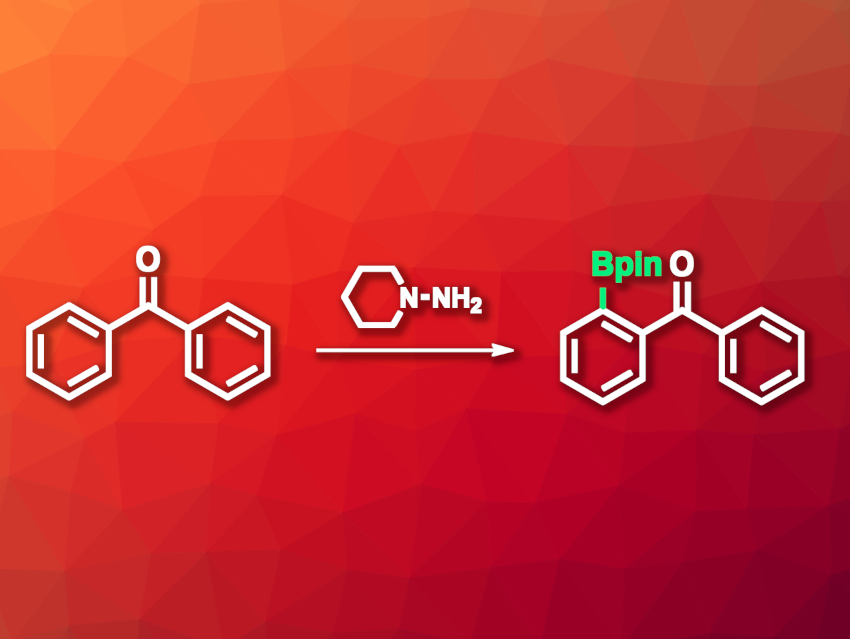
Diarylketones, or benzophenone derivatives, are useful chemical building blocks. C–H borylation reactions are important transformations and can be useful, e.g., to allow the functionalization of diarylketones. However, they usually require metal catalysts. Directed, metal-free C–H borylation reactions, which are mediated by directing groups that are either permanently present in the substrate or need to be installed and removed in additional steps, can be useful as an alternative. However, this can lead to either limited substrate scopes or reduced step economy.
Gaorong Wu, Gannan Medical University, Ganzhou, China, Yafei Ji, East China University of Science and Technology, Shanghai, China, and colleagues have developed an approach to the ortho-selective borylation of benzophenones that uses hydrazones as a traceless, transient directing group. This type of directing group is automatically removed in the course of the reaction. The team reacted a range of benzophenone derivatives with N-aminopiperidine to generate hydrazones, which were reacted with BBr3 in the presence of 2,6-lutidine as a base to obtain borylated products.
The installed BBr2 group was further transformed via a reaction with pinacol, removing the directing group and giving aryl pinacol boronates. This reaction can be combined with further transformations in a one-pot approach, such as oxidations, chlorinations, brominatios, and Suzuki–Miyaura coupling reactions. According to the researchers, the work could provide a tool for synthesizing diverse benzophenone structures.
- C–H Borylation of Benzophenones Using Hydrazone as the Traceless Directing Group,
Zhaoziyuan Yang, Liqiang Hao, Xiaobo Xu, Yangyang Wang, Gaorong Wu, Yafei Ji,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02142