The research tackles the challenge of finding effective α-glucosidase inhibitors, which are important for managing type 2 diabetes. Current drugs like acarbose have limitations, so new molecules with better activity and fewer side effects are needed.
The study was conducted by a team of researchers, including Kashmiri Lal, University of Science & Technology, Hisar, India, and colleagues. The team designed adamantane-based 1,2,3-triazole hybrids, a unique molecular framework. These hybrids showed strong α-glucosidase inhibition, outperforming the standard drug acarbose in some cases.
This study outlines the synthesis of several adamantane-functionalized 1,2,3-triazoles through the copper-catalyzed azide-alkyne cycloaddition (CuAAC), “click chemistry.” The starting alkyne was reacted with three types of azides: benzyl azides (3a–3f), generated in situ from benzyl bromides and NaN₃; phenyl azides (5a–5f), synthesized by diazotizing aromatic amines followed by azidation with NaN₃ in DCM; and 2-azido-N-phenylacetamides (7a–7f), prepared in situ from 2-bromo-N-phenylacetamides and NaN₃. The final products were 1,2,3-triazole hybrids (4a-4f, 6a-6f and 8a-8f).
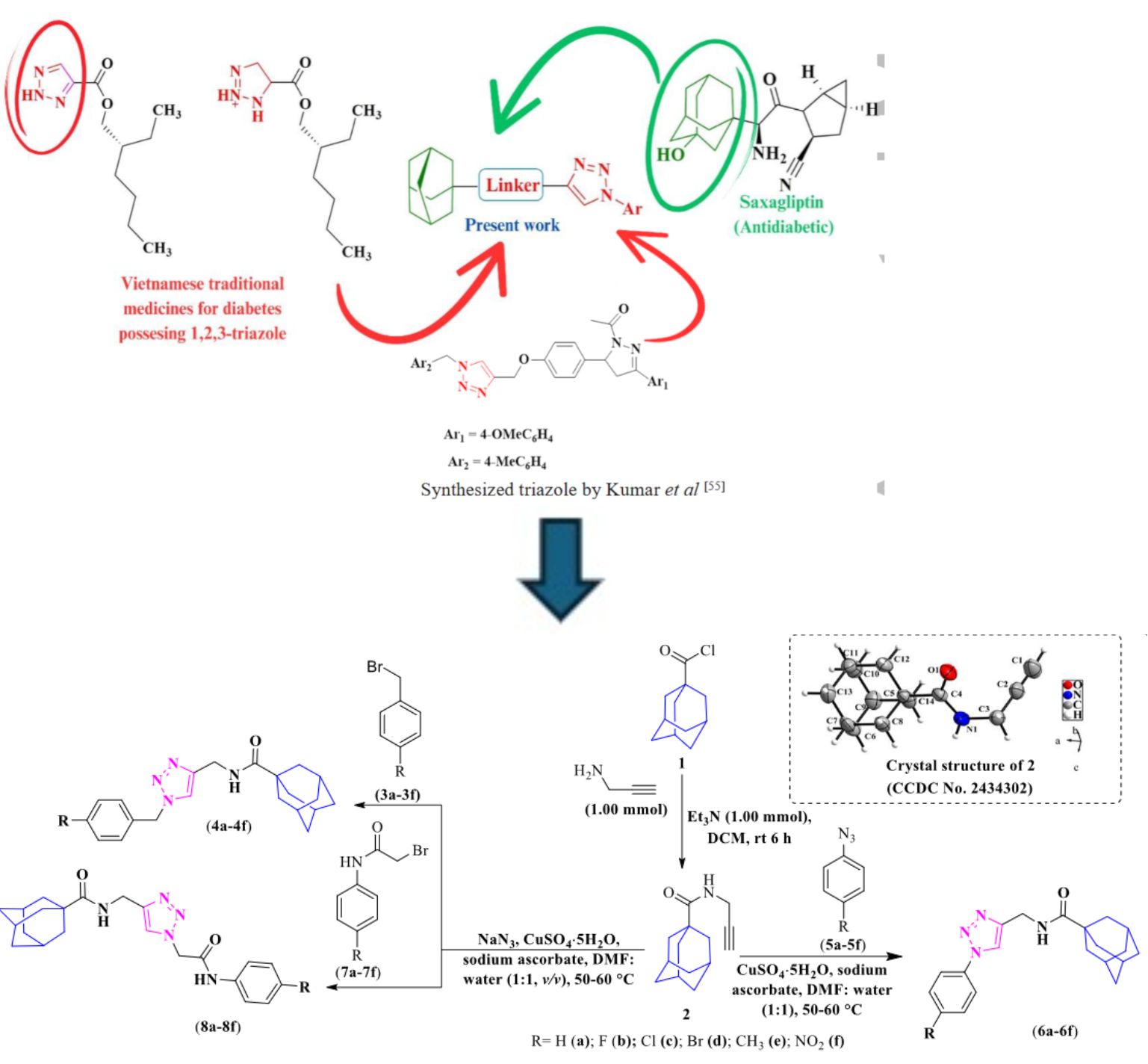
To evaluate their potential as antidiabetic agents, all compounds were tested for α-glucosidase inhibition, and structure-activity relationship (SAR) analysis was performed to identify chemical features linked to enhanced activity. Computational studies complemented the experimental work, including molecular docking simulations with the target protein (PDB ID: 3L4U), followed by 100 ns molecular dynamics simulations to assess binding stability. Binding energies were computed using MMGBSA and MMPBSA methods, with compound 6c showing a binding energy of –15.74 kcal/mol, compared to –46.37 kcal/mol for the standard drug acarbose. Furthermore, drug-likeness evaluations based on Lipinski’s Rule of Five indicated promising oral bioavailability across the synthesized compounds.
The key finding of the study is that two synthesized compounds, 6c and 6b, demonstrated strong α-glucosidase inhibitory activity, which is promising for antidiabetic therapy. Specifically, compound 6c showed the most potent effect with an IC₅₀ value of 8.30 µM, outperforming the standard drug acarbose (IC₅₀ of 13.50 µM). Compound 6b also exhibited notable activity with an IC₅₀ of 14.0 µM. Additionally, molecular docking and simulation studies revealed that compound 6c had strong binding affinity and stable interactions with the target enzyme, highlighting its potential as a lead compound for further development.
This study opens the door to new antidiabetic drug candidates based on adamantane-triazole scaffolds. The promising results suggest these molecules could be optimized further and tested in preclinical models. It’s a step toward safer, more effective treatments for diabetes.
- Adamantane Appended 1,2,3-Triazole Hybrids: Synthesis and α-Glucosidase Inhibition Studies Through Experimental and In Silico Approach,
Aman Ragshaniya, Subhadip Maity, Lokesh Kumar, Vivek Asati, Poojita Poojita, Avijit Kumar Paul, Jayant Sindhu, Kashmiri Lal,
ChemMedChem 2025.
https://doi.org/10.1002/cmdc.202500263