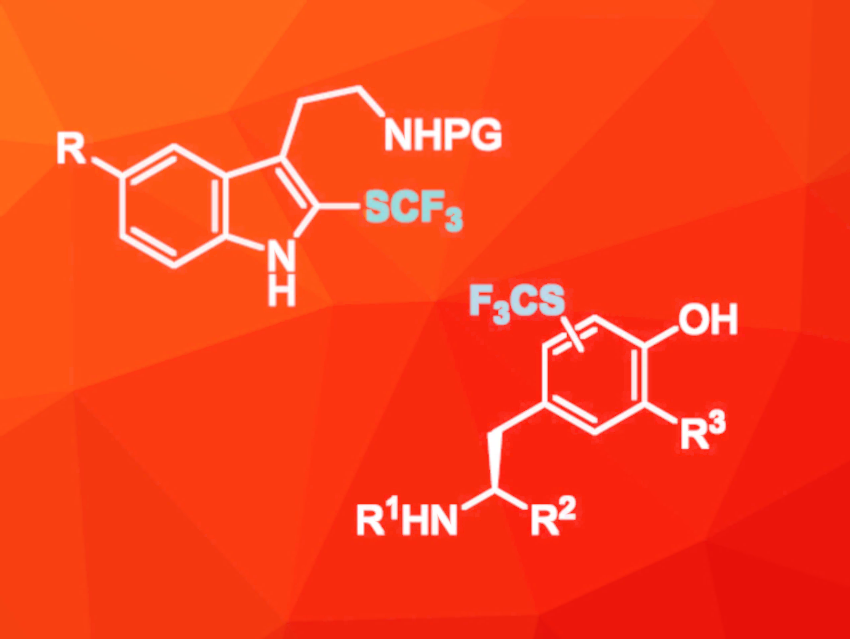
Many pharmaceutically active compounds contain fluorine atoms because this often leads to favorable physicochemical properties. In the case of peptide-based drugs, fluorine-containing groups can increase the hydrophobicity of the peptides, which makes it easier for them to get through cell walls. Additionally, fluorine atoms in biomolecules can serve as probes for 19F NMR spectroscopy. Methods for the introduction of single fluorine atoms or trifluoromethyl groups are well-established, but the synthesis of amino acids containing other fluorinated groups, such as trifluoromethylthio groups (SCF3), is less well explored.
Grégory Chaume, Thierry Brigaud, Université Paris-Saclay, Orsay, France, Jerney Iskra, University of Ljubljana, Slovenia, and colleagues have developed a method for the trifluoromethylthiolation of the amino acids tryptophane and tyrosine and their derivatives. The amino acids or amines were reacted with an electrophilic trifluoromethanesulfenamide in the presence of triflic acid or BF3·OEt2. Depending on the reactivity of the starting materials, the reaction was either carried out in dichloromethane at room temperature or in 1,2-dichloroethane at 50 °C.
The target products were obtained in good to high yields of up to 97 %, and the reaction also worked well on a multi-gram scale. The modified amino acids were also suitable for use in solid-phase peptide synthesis. The procedure was successfully used for the late-stage functionalization of short peptides, i.e., the peptide endomorphin-1 (EM-1). The modified forms of EM-1 showed longer retention times during reverse-phase HPLC which indicates higher hydrophobicity. The introduction of the trifluoromethylthio group to tyrosine also leads to a 100-fold increase in the acidity of its OH group. These results demonstrate that the introduction of trifluoromethylthio groups can be used to tune the properties of amino acids and the resulting peptides.
- Trifluoromethylthiolation of Tryptophan and Tyrosine Derivatives: A Tool for Enhancing the Local Hydrophobicity of Peptides,
Jure Gregorc, Nathalie Lensen, Grégory Chaume, Jernej Iskra, Thierry Brigaud,
J. Org. Chem. 2023.
https://doi.org/10.1021/acs.joc.3c01373