Thermally activated delayed fluorescence (TADF) molecules are promising materials for high-performance organic light-emitting diodes (OLEDs). The linker groups connecting donor (D) and acceptor (A) units in D-A type TADF molecules could affect the charge transfer and luminescence performance of TADF materials in aggregated states.
Peng Wu, Zixing Wang, Shanghai University, China, Stefan Bräse, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have developed four TADF molecules that feature an aza-azulene donor (pictured in red), a 4,6-diphenyl-1,3,5-triazine acceptor (pictured in orange) and either planar or twisted linkers (pictured in dark blue). Phenanthrene and triphenylene were used as planar linkers, and 1-phenylnaphthalene and 1,1′-binaphthalene were used as twisted linkers. The target molecules were prepared via nucleophilic substitution reactions in yields of 65–72 %, and all four show TADF features.
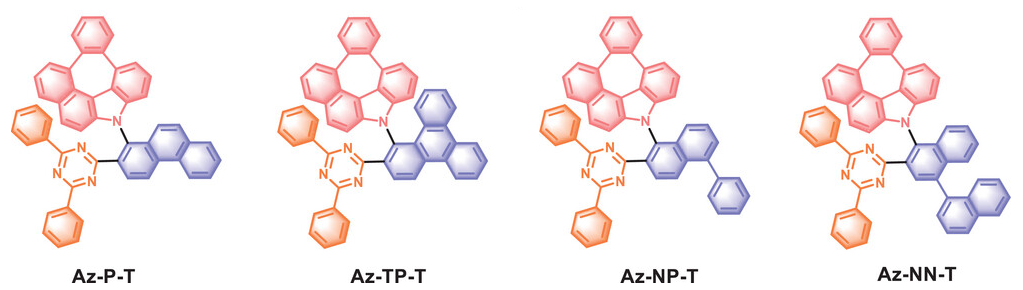
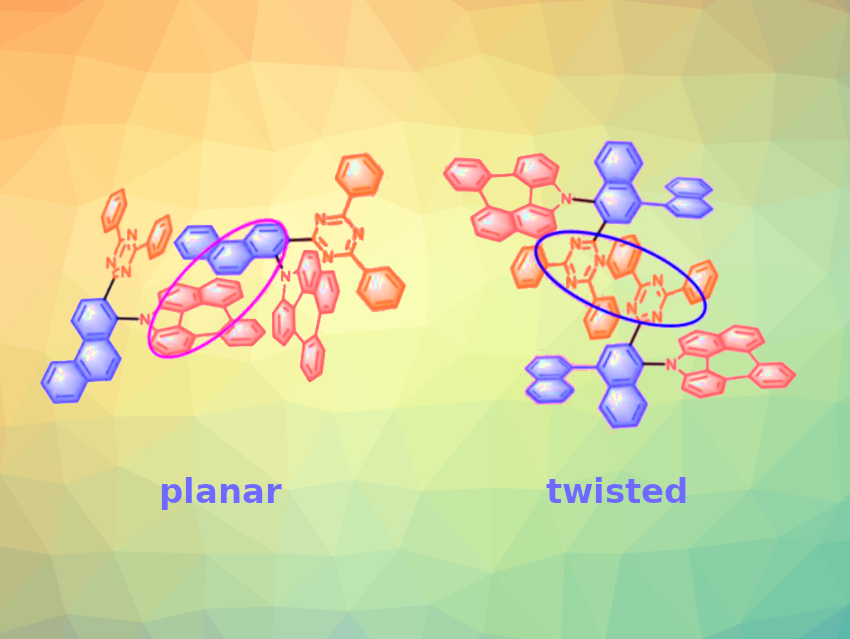
The team found that Az-NP-T and Az-NN-T, which contain twisted linkers, exhibit a particularly balanced hole- and electron-transport ability. This is due to improved acceptor stacking induced by the twisted linker: Compared with planar linkers, twisted linkers can promote the formation of better π-stacking between the triazine acceptor groups of adjacent molecules, resulting in an additional channel for electron transport. The team used the new TADF molecules to build OLEDs and obtained highly efficient and stable devices with deep-red emission.
- Linker aggregation engineering of TADF materials to tune carrier balance for highly efficient organic LEDs with long operational lifetime,
Zhen Zhang, Rongrong Xia, Ke Wang, Youjun Wu, Panpan Zang, Xuemin Gan, Zhangcheng Liao, Bin Wei, Peng Wu, Stefan Bräse, Zixing Wang,
Aggregate 2024.
https://doi.org/10.1002/agt2.588



Importante