The research was conducted by a team of scientists led by Larry L. David, Oregon Health & Science University, Portland, USA, Kirsten J. Lampi, Oregon Health & Science University, Portland, USA, and colleagues, known for their work in protein chemistry and mass spectrometry.
This is the first comprehensive mapping of isomerized, racemized, and deamidated residues in γS-crystallin from aged human lenses. It reveals how non-enzymatic chemical changes accumulate in long-lived proteins and potentially disrupt their function, offering new insights into protein aging and cataract development.
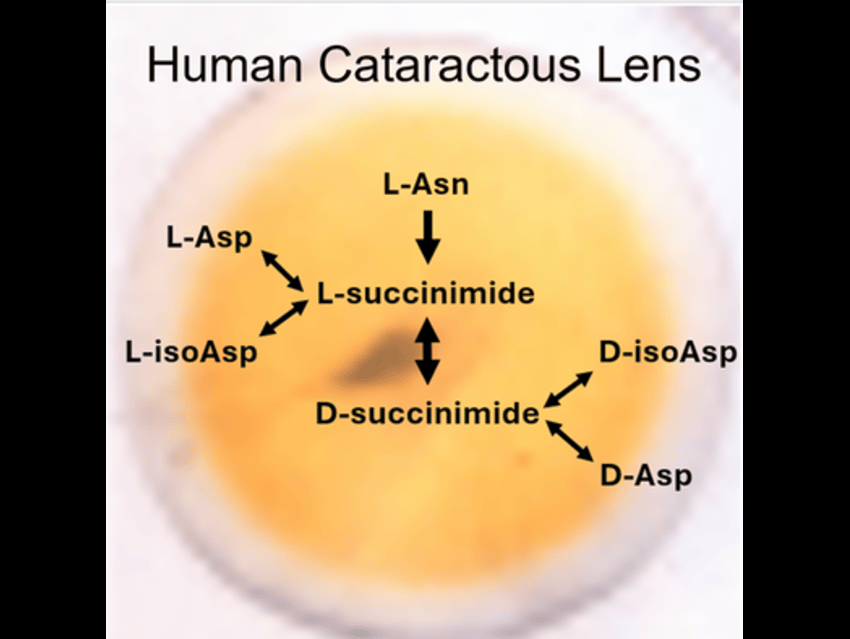
The researchers used high-resolution mass spectrometry to analyze γS-crystallin extracted from aged human lenses, employing a sequence of steps that included protein extraction and enzymatic digestion, liquid chromatography for peptide separation, and mass spectrometry to detect subtle mass changes caused by chemical modifications. Detailed data analysis then pinpointed specific sites of isomerization, racemization, and deamidation. These modifications are driven by spontaneous intramolecular reactions: deamidation of asparagine yields either aspartate or isoaspartate, while isomerization and racemization of aspartate produce isoaspartate and D-aspartate. Notably, these processes often involve a succinimide intermediate and are facilitated under physiological pH and temperature conditions.
The researchers discovered that specific regions of the protein, γS-crystallin, contain isomerized and racemized aspartyl residues as well as deamidated asparagine residues. These modifications occur in areas essential for the protein’s structural stability and function, suggesting they could weaken the protein and contribute to age-related vision problems like cataracts.
By mapping these modifications, the study paves the way for potential treatments to slow or reverse protein aging, early diagnostic tools to detect lens changes before vision is affected, and further research into age-related damage in other long-lived proteins tied to degenerative diseases.
- Isomerized and Racemized Aspartyl and Deamidated Asparagine Residues Identified in ɣS-Crystallin,
Victoria S. Halls, Larry L. David, Keith D. Zientek, Kirsten J. Lampi,
ChemBioChem 2025.
https://doi.org/10.1002/cbic.202500352