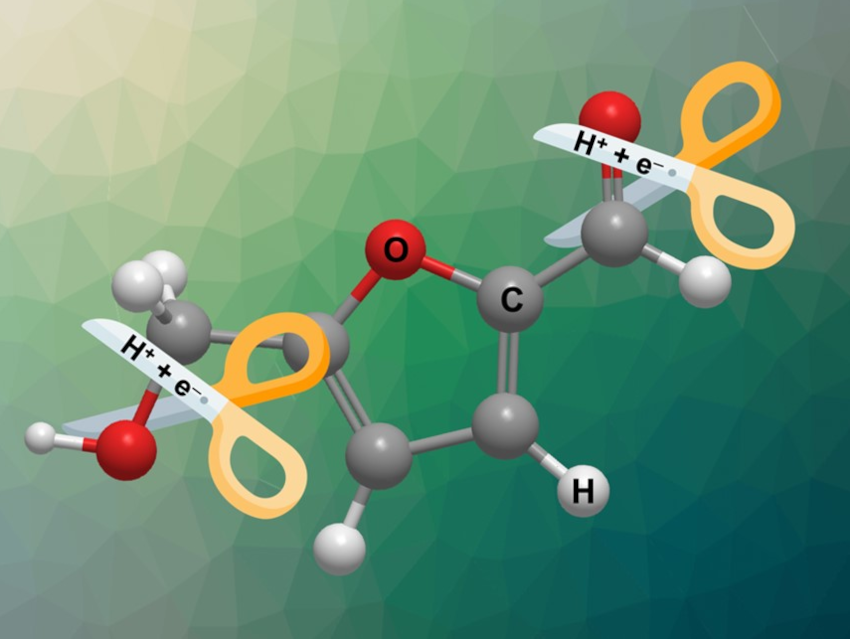
Hydrogenation and hydrogenolysis are two important reactions for the electrochemical reductive upgrading of biomass-derived compounds such as 5-hydroxymethylfurfural. Decreasing pH promotes hydrogenolysis (which combines hydrogenation and deoxygenation) over hydrogenation (which does not involve the C–O bond cleavage). However, so far there had been no mechanistic understanding to explain this observed pH effect on hydrogenolysis. Identifying factors that can promote hydrogenolysis would be useful for the valorization of biomass-derived, oxygen-containing compounds.
Kyoung-Shin Choi, University of Wisconsin-Madison, Madison, USA, and colleagues have combined experimental and computational methods to investigate the mechanistic differences between electrochemical hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural. The team used nanocrystalline copper foam electrodes for the electrochemical reaction and observed the product distributions of the reduction of 5-hydroxymethylfurfural at different pH values. Calculations were used to find plausible pathways and intermediates that could explain why the hydrogenolysis is promoted at low pH values.
The team found that the hydrogenation and hydrogenolysis of aldehydes are competing reactions that involve the formation of different surface-adsorbed intermediates via different mechanisms. The key surface-adsorbed intermediates for hydrogenolysis are formed via a proton-coupled electron transfer, which is kinetically feasible only with a high proton concentration in solution—i.e., at low pH values. In contrast, the intermediates for hydrogenation can be formed via hydrogen-atom transfer, which is largely unaffected by pH. This work explains the origin of the pH effect on hydrogenolysis and hydrogenation.
- Mechanistic Differences between Electrochemical Hydrogenation and Hydrogenolysis of 5‐Hydroxymethylfurfural and Their pH Dependence,
Xin Yuan, Kwanpyung Lee, Michael T. Bender, J.R. Schmidt, Kyoung-Shin Choi,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202200952