Organic compounds with donor–acceptor bonds can form interesting chemical species, e.g., involving low-valent main-group elements. Carbodiphosphoranes, for example, are considered carbon(0) compounds with two donor–acceptor bonds of the type R3P→C(0)←PR3. In addition to carbon, other main-group elements (E) can also form compounds of the type L→E←L. Compounds of the type L→N+←L, for example, would be isoelectronic to carbodicarbenes.
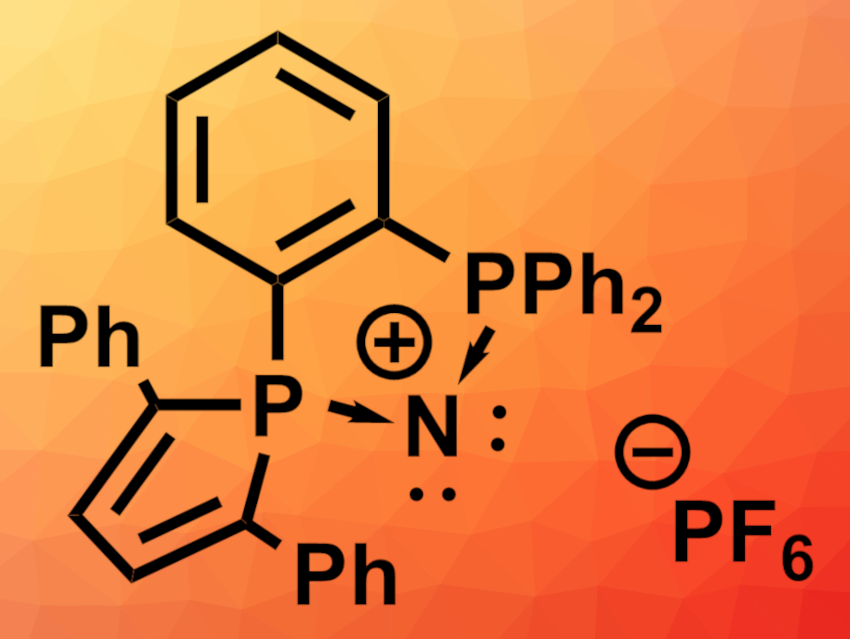
Audrey Auffrant, Institut Polytechnique de Paris, Palaiseau, France, Vincent Gandon, Université Paris-Saclay, Orsay, France, and Institut Polytechnique de Paris, and colleagues have synthesized a rare type of diphosphazenium cation (pictured). This cyclic compound is proposed to be an example of a divalent N(I) species that is isoelectronic to carbodiphosphoranes.
The team started from [PPh3–NHtBu]Br, which was lithiated using BuLi and reacted with 1-cyanophosphole-2,5-diphenylphosphole to give a precursor for the phosphorus ligand. This precursor was deprotonated using potassium hexamethyldisilazane (KHDMS) to give a phosphole-iminophosphorane derivative. A reaction with AgPF6 then gave the diphosphazenium salt.
The structure of the product was confirmed using X-ray diffraction analysis. Density functional theory (DFT) calculations were used to investigate the bonding situation. The team found that the product can be best described as a divalent N(I) species with two lone pairs at the nitrogen atom (pictured). Thus, the compound is isoelectronic to carbodiphosphoranes.
- A cyclic divalent N(i) species isoelectronic to carbodiphosphoranes,
Jiaxin Tian, Marie Cordier, Christophe Bour, Audrey Auffrant, Vincent Gandon,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc01637k