Martin Jansen, Max Planck Institute for Solid State Research, Stuttgart, Germany, Sebastian Riedel, Free University Berlin, Germany, and colleagues have observed an unexpected reaction while investigating the chemistry of ozonides.
The team aimed to use crystal engineering to force ozonide ions close together in the solid phase and possibly induce dimerization. They used the partially fluorinated aniline-based cation [NMe3(C6H3(CF3)2)]+ for this. However, they found that solutions of [NMe3(C6H3(CF3)2)]O3 in liquid ammonia, which were prepared using KO3, change color spontaneously, indicating an unintended reaction.
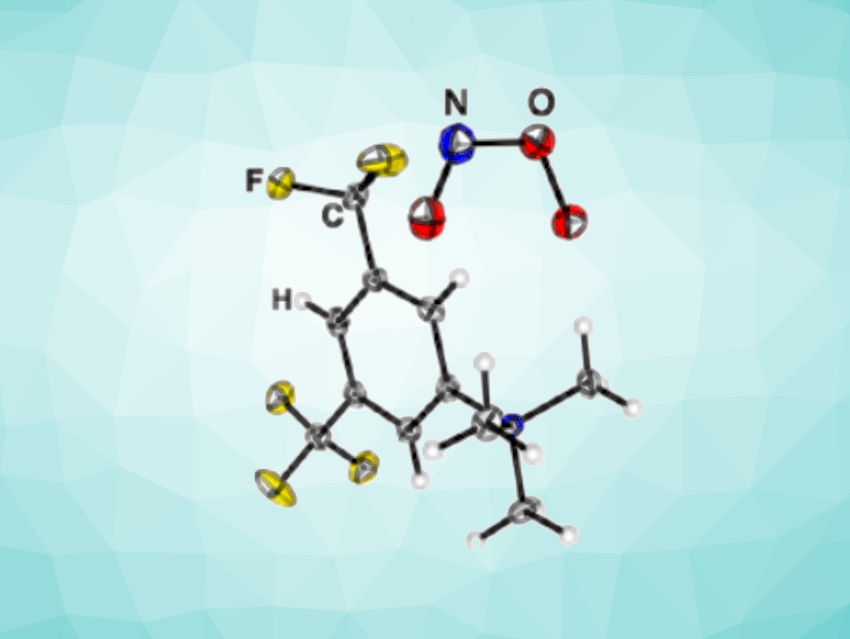
The researchers evaporated the solvent and obtained crystals of the peroxynitrite [NMe3(C6H3(CF3)2)][ONOO]•1/3 NH3. This allowed them to obtain high-resolution structural data of the peroxynitrite anion in a cis-conformation for the first time. The ion was also studied by single-crystal Raman spectroscopy, and the properties of the cis and trans isomers of peroxynitrite were analyzed computationally. The team also tested different cations and found that fluorine-specific interactions play a key role in the unexpected formation of peroxynitrite.
- Formation of Peroxynitrite, [O‐N‐O‐O]–, via a Cascade of Reactions between Ozonide and Ammonia,
Jonas R. Schmid, Oliver R. H. Rennefeld, Anja Wiesner, Martin Jansen, Sebastian Hasenstab-Riedel,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202400585