Metal–organic frameworks (MOFs) are porous materials composed of metal centers and organic linkers. They have applications, e.g., in gas separation or catalysis. The materials could be useful, e.g., for the capture of the greenhouse gas CO2—or, instead of simply capturing CO2, it could be turned into carboxylates and used in the synthesis of MOFs.
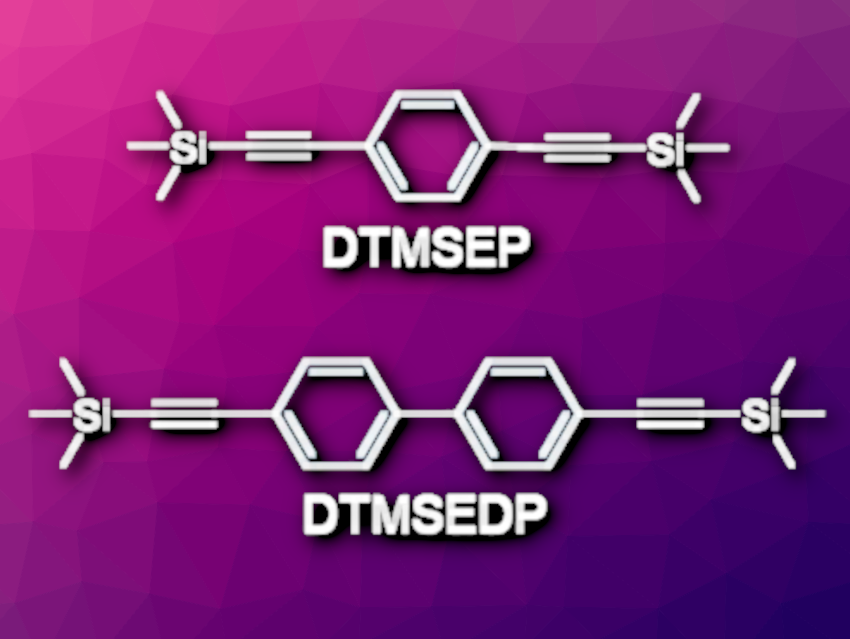
Bin Liu, National University of Singapore, and colleagues have used CO2 to synthesize two stable, zirconium-based MOFs via a sequential desilylation–carboxylation–coordination reaction. The team first used Sonogashira coupling reactions to prepare the alkynylsilane building blocks DTMSEP and DTMSEDP (pictured). These building blocks were then reacted with CO2 in the presence of cesium fluoride in N,N-dimethylformamide (DMF). C–Si bonds were cleaved (promoted by CsF), and CO2 inserted into the terminal alkynes, ultimately giving dipropionic acids.
The researchers then used zirconium(IV) oxide chloride octahydrate (ZrOCl2·8H2O) to prepare two MOFs, called CO2–Zr-DEP and CO2–Zr-DEDP, from the dipropionic acids. The team also loaded Ag(I) ions into the Zr-based MOFs, obtaining heterogeneous catalysts for the efficient conversion of CO2 and propargylic alcohols to cyclic carbonates. Thus, they have developed a dual-pronged strategy for CO2 utilization.
- CO2-Based Stable Porous Metal–Organic Frameworks for CO2 Utilization,
Bo Song, Yuhang Liang, Yi Zhou, Liang Zhang, He Li, Neng-Xiu Zhu, Ben Zhong Tang, Dan Zhao, Bin Liu,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c03476